Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study
- PMID: 28223062
- PMCID: PMC5809128
- DOI: 10.1016/S1470-2045(17)30104-3
Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study
Abstract
Background: Squamous cell carcinoma of the anal canal (SCCA) is a rare malignancy associated with infection by human papillomavirus (HPV). No consensus treatment approach exists for the treatment of metastatic disease. Because intratumoral HPV oncoproteins upregulate immune checkpoint proteins such as PD-1 to evade immune-mediated cytotoxicity, we did a trial of the anti-PD-1 antibody nivolumab for patients with metastatic SCCA.
Methods: We did this single-arm, multicentre, phase 2 trial at ten academic centres in the USA. We enrolled patients with treatment-refractory metastatic SCCA, who were given nivolumab every 2 weeks (3 mg/kg). The primary endpoint was response according to Response Evaluation Criteria in Solid Tumors, version 1.1, in the intention-to-treat population. At the time of data cutoff, the study was ongoing, with patients continuing to receive treatment. The study is registered with ClinicalTrials.gov, number NCT02314169.
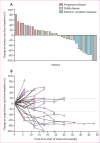
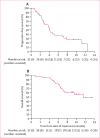
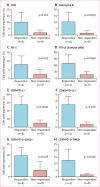
Results: We screened 39 patients, of whom 37 were enrolled and received at least one dose of nivolumab. Among the 37 patients, nine (24% [95% CI 15-33]) had responses. There were two complete responses and seven partial responses. Grade 3 adverse events were anaemia (n=2), fatigue (n=1), rash (n=1), and hypothyroidism (n=1). No serious adverse events were reported.
Interpretation: To our knowledge, this is the first completed phase 2 trial of immunotherapy for SCCA. Nivolumab is well tolerated and effective as a monotherapy for patients with metastatic SCCA. Immune checkpoint blockade appears to be a promising approach for patients with this orphan disease.
Funding: National Cancer Institute/Cancer Therapy Evaluation Program, the HPV and Anal Cancer Foundation, the E B Anal Cancer Fund, The University of Texas MD Anderson Moon Shots Program, and an anonymous philanthropic donor.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
PSi has received fees from Bayer Pharmaceuticals and personal fees from Merrimack Pharma. EC has received grants from National Cancer Institute to her institution, and personal fees for advisory boards from Advaxis, Lilly, Merrimack, Bayer, Taiho, and Amgen. MP has received grants from the National Cancer Institute/Cancer Therapy Evaluation Program. KB has received personal fees from Guardant Health. RL has received personal fees from Guardant Health. JA owns stock in, and has acted as a consultant or on advisory boards for, Jounce, Kite Pharma, Evelo, Constellation, Bristol-Myers Squibb, GlaxoSmithKline, AstraZeneca, Amgen, and Neon. PSh owns stock in, and has acted as a consultant for, Jounce, Kite Pharma, Neon, Bristol-Myers Squibb, GlaskoSmithKline, AstraZeneca, Amgen, Constellation, Evelo. CE has received grants from Daiichi and Keryx, and personal fees from Bayer, Sirtex, Genentech, Roche/Genentech, and Bayer. VKM, MES, HN, SI, KC, BP, DD, JLW, LX, TB-S, LV, JB, AM, WCF, CO, GB, AO, JR, AM, RAW, and HS declare no completing interests.
Figures
Comment in
-
Anal cancer: from an orphan disease to a curable malignancy?Lancet Oncol. 2017 Apr;18(4):415-416. doi: 10.1016/S1470-2045(17)30091-8. Epub 2017 Feb 20. Lancet Oncol. 2017. PMID: 28223061 No abstract available.
-
[Nivolumab for pretreated metastatic anal cancer : Immune checkpoint blockade is also advised in combination with radiochemotherapy].Strahlenther Onkol. 2018 Apr;194(4):356-357. doi: 10.1007/s00066-018-1272-8. Strahlenther Onkol. 2018. PMID: 29392353 German. No abstract available.
References
-
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. - PubMed
-
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–49. - PubMed
-
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. - PubMed
-
- Das P, Bhatia S, Eng C, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68:794–800. - PubMed
-
- Eng C. Anal cancer: current and future methodology. Cancer Invest. 2006;24:535–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

