Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment
- PMID: 28223486
- PMCID: PMC5347541
- DOI: 10.1073/pnas.1616070114
Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment
Abstract
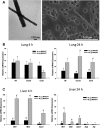
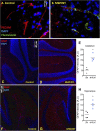
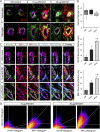
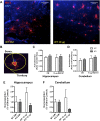
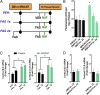
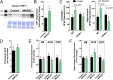
Pulmonary exposure to multiwalled carbon nanotubes (MWCNTs) causes indirect systemic inflammation through unknown pathways. MWCNTs translocate only minimally from the lungs into the systemic circulation, suggesting that extrapulmonary toxicity may be caused indirectly by lung-derived factors entering the circulation. To assess a role for MWCNT-induced circulating factors in driving neuroinflammatory outcomes, mice were acutely exposed to MWCNTs (10 or 40 µg/mouse) via oropharyngeal aspiration. At 4 h after MWCNT exposure, broad disruption of the blood-brain barrier (BBB) was observed across the capillary bed with the small molecule fluorescein, concomitant with reactive astrocytosis. However, pronounced BBB permeation was noted, with frank albumin leakage around larger vessels (>10 µm), overlain by a dose-dependent astroglial scar-like formation and recruitment of phagocytic microglia. As affirmed by elevated inflammatory marker transcription, MWCNT-induced BBB disruption and neuroinflammation were abrogated by pretreatment with the rho kinase inhibitor fasudil. Serum from MWCNT-exposed mice induced expression of adhesion molecules in primary murine cerebrovascular endothelial cells and, in a wound-healing in vitro assay, impaired cell motility and cytokinesis. Serum thrombospondin-1 level was significantly increased after MWCNT exposure, and mice lacking the endogenous receptor CD36 were protected from the neuroinflammatory and BBB permeability effects of MWCNTs. In conclusion, acute pulmonary exposure to MWCNTs causes neuroinflammatory responses that are dependent on the disruption of BBB integrity.
Keywords: blood-brain barrier; microglia; multiwalled carbon nanotube; nanoparticle; thrombospondin-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

