Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models
- PMID: 28223512
- PMCID: PMC5347608
- DOI: 10.1073/pnas.1616152114
Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models
Abstract
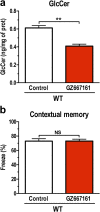
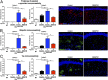
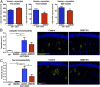
Mutations in the glucocerebrosidase gene (GBA) confer a heightened risk of developing Parkinson's disease (PD) and other synucleinopathies, resulting in a lower age of onset and exacerbating disease progression. However, the precise mechanisms by which mutations in GBA increase PD risk and accelerate its progression remain unclear. Here, we investigated the merits of glucosylceramide synthase (GCS) inhibition as a potential treatment for synucleinopathies. Two murine models of synucleinopathy (a Gaucher-related synucleinopathy model, GbaD409V/D409V and a A53T-α-synuclein overexpressing model harboring wild-type alleles of GBA, A53T-SNCA mouse model) were exposed to a brain-penetrant GCS inhibitor, GZ667161. Treatment of GbaD409V/D409V mice with the GCS inhibitor reduced levels of glucosylceramide and glucosylsphingosine in the central nervous system (CNS), demonstrating target engagement. Remarkably, treatment with GZ667161 slowed the accumulation of hippocampal aggregates of α-synuclein, ubiquitin, and tau, and improved the associated memory deficits. Similarly, prolonged treatment of A53T-SNCA mice with GZ667161 reduced membrane-associated α-synuclein in the CNS and ameliorated cognitive deficits. The data support the contention that prolonged antagonism of GCS in the CNS can affect α-synuclein processing and improve behavioral outcomes. Hence, inhibition of GCS represents a disease-modifying therapeutic strategy for GBA-related synucleinopathies and conceivably for certain forms of sporadic disease.
Keywords: GBA mutations; Gaucher disease; Lewy body dementia; Parkinson’s disease; glucosylceramide synthase.
Conflict of interest statement
Conflict of interest statement: S.P.S., C.V., J.C., C.M.T., A.M.R, H.P., M.A.O., J.C.D., J.M., E.M., B.W., S.H.C., and L.S.S. are employees of Sanofi.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

