Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis
- PMID: 28223524
- PMCID: PMC5347553
- DOI: 10.1073/pnas.1701529114
Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis
Abstract
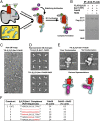
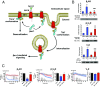
β-Arrestins (βarrs) interact with G protein-coupled receptors (GPCRs) to desensitize G protein signaling, to initiate signaling on their own, and to mediate receptor endocytosis. Prior structural studies have revealed two unique conformations of GPCR-βarr complexes: the "tail" conformation, with βarr primarily coupled to the phosphorylated GPCR C-terminal tail, and the "core" conformation, where, in addition to the phosphorylated C-terminal tail, βarr is further engaged with the receptor transmembrane core. However, the relationship of these distinct conformations to the various functions of βarrs is unknown. Here, we created a mutant form of βarr lacking the "finger-loop" region, which is unable to form the core conformation but retains the ability to form the tail conformation. We find that the tail conformation preserves the ability to mediate receptor internalization and βarr signaling but not desensitization of G protein signaling. Thus, the two GPCR-βarr conformations can carry out distinct functions.
Keywords: GPCR; arrestin; desensitization; endocytosis; signaling.
Conflict of interest statement
Conflict of interest statement: R.J.L. is a cofounder and shareholder of Trevena.
Figures





References
-
- Lefkowitz RJ. The superfamily of heptahelical receptors. Nat Cell Biol. 2000;2(7):E133–E136. - PubMed
-
- Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture) Angew Chem Int Ed Engl. 2013;52(25):6366–6378. - PubMed
-
- Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. - PubMed
-
- Moore CAC, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. - PubMed
-
- Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

