Thymol mitigates lipopolysaccharide-induced endometritis by regulating the TLR4- and ROS-mediated NF-κB signaling pathways
- PMID: 28223539
- PMCID: PMC5386742
- DOI: 10.18632/oncotarget.15373
Thymol mitigates lipopolysaccharide-induced endometritis by regulating the TLR4- and ROS-mediated NF-κB signaling pathways
Abstract
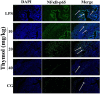
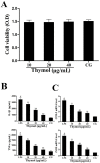
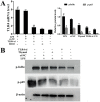
The purpose of this study was to investigate the effects of thymol on lipopolysaccharide (LPS)-induced inflammatory responses and to clarify the potential mechanism of these effects. LPS-induced mouse endometritis was used to confirm the anti-inflammatory action of thymol in vivo. RAW264.7 cells were used to examine the molecular mechanism and targets of thymol in vitro. In vivo, thymol markedly alleviated LPS-induced pathological injury, myeloperoxidase (MPO) activity, and the production of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in mice. Further studies were performed to examine the expression of the Toll-like receptor 4 (TLR4) -mediated nuclear factor-κB (NF-κB) pathway. These results showed that the expression of the TLR4-mediated NF-κB pathway was inhibited by thymol treatment. In vitro, we observed that thymol dose-dependently inhibited the expression of TNF-α, IL-1β, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) in LPS-stimulated RAW264.7 cells. Moreover, the results obtained from immunofluorescence assays also indicated that thymol dose-dependently suppressed LPS-induced reactive oxygen species (ROS) production. Silencing of TLR4 inhibited NF-κB pathway activation. Furthermore, H2O2 treatment increased the phosphorylation of p65 and IκBα, which were decreased when treated with N-acetyl cysteine or thymol. In conclusion, the anti-inflammatory effects of thymol are associated with activation of the TLR4 or ROS signaling pathways, contributing to NF-κB activation, thereby alleviating LPS-induced oxidative and inflammatory responses.
Keywords: TLR4; inflammation; nuclear factor-κB; reactive oxygen species; thymol.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






References
-
- Eslami M, Bolourchi M, Seifi HA, Asadi F, Akbari R. Treatment of clinical endometritis in dairy cows by previously used controlled internal drug release devices. Theriogenology. 2015;84:437–445. - PubMed
-
- Sheldon IM, Price SB, Cronin J, Gilbert RO, Gadsby JE. Mechanisms of infertility associated with clinical and subclinical endometritis in high producing dairy cattle. Reproduction in domestic animals. 2009;44(Suppl 3):1–9. - PubMed
-
- Luo J, Xu Y, Zhang M, Gao L, Fang C, Zhou C. Magnolol inhibits LPS-induced inflammatory response in uterine epithelial cells : magnolol inhibits LPS-induced inflammatory response. Inflammation. 2013;36:997–1003. - PubMed
-
- Swangchan-Uthai T, Lavender CR, Cheng Z, Fouladi-Nashta AA, Wathes DC. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biology of reproduction. 2012;87:135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

