Neuronal plasticity of trigeminal ganglia in mice following nerve injury
- PMID: 28223844
- PMCID: PMC5310634
- DOI: 10.2147/JPR.S120092
Neuronal plasticity of trigeminal ganglia in mice following nerve injury
Abstract
Background: Nerve injury may induce neuropathic pain. In studying the mechanisms of orofacial neuropathic pain, attention has been paid to the plastic changes that occur in the trigeminal ganglia (TGs) and nucleus in response to an injury of the trigeminal nerve branches. Previous studies have explored the impact of sciatic nerve injury on dorsal root ganglia (DRGs) and it has shown dramatic changes in the expression of multiple biomarkers. In large, the changes in biomarker expression in TGs after trigeminal nerve injury are similar to that in DRGs after sciatic nerve injury. However, important differences exist. Therefore, there is a need to study the plasticity of biomarkers in TGs after nerve injury in the context of the development of neuropathic pain-like behaviors.
Aim: The aim of this study was to investigate the plasticity of biomarkers associated with chronic persistent pain in TGs after trigeminal nerve injury.
Materials and methods: To mimic the chronic nature of the disorder, we used an intraoral procedure to access the infraorbital nerve (ION) and induced a nerve injury in mice. Immunohistochemistry and quantification were used for revealing the expression level of each biomarker in TGs after nerve injury.
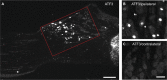
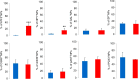
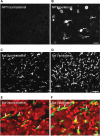
Results: Two weeks after partial ION injury, immunohistochemistry results showed strongly upregulated expressions of activating transcription factor 3 and neuropeptide Y (NPY) in the ipsilateral TGs. Microglial cells were also activated after nerve injury. In regard to positive neuronal profile counting, however, no significant difference in expression was observed in galanin, substance P, calcitonin gene-related peptide, neuronal nitric oxide synthase, phosphorylated AKT, or P2X3 in ipsilateral TGs when compared to contralateral TGs.
Conclusion: In this study, the expression and regulation of biomarkers in TGs have been observed in response to trigeminal nerve injury. Our results suggest that NPY and Iba1 might play crucial roles in the pathogenesis of orofacial neuropathic pain following this type of injury. Further investigations on the relevance of these changes may help to target suitable treatment possibilities for trigeminal neuralgia.
Keywords: animal model; infraorbital nerve; neuropeptides; orofacial pain; sensory neurons.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Potential Molecular Targets for Treating Neuropathic Orofacial Pain Based on Current Findings in Animal Models.Int J Mol Sci. 2021 Jun 15;22(12):6406. doi: 10.3390/ijms22126406. Int J Mol Sci. 2021. PMID: 34203854 Free PMC article. Review.
-
Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions.J Pain. 2008 Nov;9(11):1036-48. doi: 10.1016/j.jpain.2008.06.006. Epub 2008 Aug 16. J Pain. 2008. PMID: 18708302 Free PMC article.
-
Expression of peptides, nitric oxide synthase and NPY receptor in trigeminal and nodose ganglia after nerve lesions.Exp Brain Res. 1996 Oct;111(3):393-404. doi: 10.1007/BF00228728. Exp Brain Res. 1996. PMID: 8911933
-
Bone marrow stromal cells attenuate injury-induced changes in galanin, NPY and NPY Y1-receptor expression after a sciatic nerve constriction.Neuropeptides. 2009 Apr;43(2):125-32. doi: 10.1016/j.npep.2008.12.003. Epub 2009 Jan 24. Neuropeptides. 2009. PMID: 19168218
-
Orofacial Neuropathic Pain-Basic Research and Their Clinical Relevancies.Front Mol Neurosci. 2021 Jul 6;14:691396. doi: 10.3389/fnmol.2021.691396. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34295221 Free PMC article. Review.
Cited by
-
Histone deacetylase inhibitors prevent persistent hypersensitivity in an orofacial neuropathic pain model.Mol Pain. 2018 Jan-Dec;14:1744806918796763. doi: 10.1177/1744806918796763. Mol Pain. 2018. PMID: 30178698 Free PMC article.
-
Lipopolysaccharide-induced Trigeminal Ganglion Nerve Fiber Damage is Associated with Autophagy Inhibition.Curr Med Sci. 2023 Jun;43(3):489-495. doi: 10.1007/s11596-023-2739-0. Epub 2023 Jun 6. Curr Med Sci. 2023. PMID: 37278832
-
Antagonism of Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Trigeminal Neuropathic Pain: Study in an Animal Model.Int J Mol Sci. 2018 Oct 25;19(11):3320. doi: 10.3390/ijms19113320. Int J Mol Sci. 2018. PMID: 30366396 Free PMC article.
-
Potential Molecular Targets for Treating Neuropathic Orofacial Pain Based on Current Findings in Animal Models.Int J Mol Sci. 2021 Jun 15;22(12):6406. doi: 10.3390/ijms22126406. Int J Mol Sci. 2021. PMID: 34203854 Free PMC article. Review.
-
In Situ Hybridisation Study of Neuronal Neuropeptides Expression in Models of Mandibular Denervation with or without Inflammation: Injury Dependant Neuropeptide Plasticity.J Cytol Histol. 2019 Jun 4;9(3):10.4172/2157-7099.1000509. doi: 10.4172/2157-7099.1000509. Epub 2018 Jun 29. J Cytol Histol. 2019. PMID: 31192032 Free PMC article.
References
-
- Jeon HJ, Han SR, Park MK, Yang KY, Bae YC, Ahn DK. A novel trigemi-nal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):149–158. - PubMed
-
- Wang X, Liang H, Zhou C, Xu M, Xu L. Sensitization induces hypersensitivity in trigeminal nerve. Clin Exp Allergy. 2012;42(11):1638–1642. - PubMed
-
- Shi TJ, Huang P, Mulder J, Ceccatelli S, Hokfelt T. Expression of p-Akt in sensory neurons and spinal cord after peripheral nerve injury. Neurosignals. 2009;17(3):203–212. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

