Nilotinib first-line therapy in patients with Philadelphia chromosome-negative/BCR-ABL-positive chronic myeloid leukemia in chronic phase: ENEST1st sub-analysis
- PMID: 28224300
- PMCID: PMC5486575
- DOI: 10.1007/s00432-017-2359-9
Nilotinib first-line therapy in patients with Philadelphia chromosome-negative/BCR-ABL-positive chronic myeloid leukemia in chronic phase: ENEST1st sub-analysis
Abstract
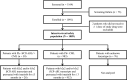
Purpose: The ENEST1st sub-analysis presents data based on Philadelphia chromosome (Ph) status, i.e., Ph+ and Ph-/BCR-ABL1 + chronic myeloid leukemia.
Methods: Patients received nilotinib 300 mg twice daily, up to 24 months.
Results: At screening, 983 patients were identified as Ph+ and 30 patients as Ph-/BCR-ABL + based on cytogenetic and RT-PCR assessment; 76 patients had unknown karyotype (excluded from this sub-analysis). In the Ph-/BCR-ABL1 + subgroup, no additional chromosomal aberrations were reported. In the Ph+ subgroup, 952 patients had safety and molecular assessments. In the Ph-/BCR-ABL1 + subgroup, 30 patients had safety assessments and 28 were followed up for molecular assessments. At 18 months, the molecular response (MR) 4 rate [MR4; BCR-ABL1 ≤0.01% on International Scale (IS)] was similar in the Ph-/BCR-ABL1+ (39.3%) and Ph+ subgroups (38.1%). By 24 months, the cumulative rates of major molecular response (BCR-ABL1IS ≤0.1%;), MR4, and MR4.5 (BCR-ABL1IS ≤0.0032%) were 85.7, 60.7, and 50.0%, respectively, in the Ph-/BCR-ABL1 + subgroup, and 80.3, 54.7, and 38.3%, respectively, in the Ph+ subgroup. In both Ph-/BCR-ABL1 + and Ph+ subgroups, rash (20 and 22%), pruritus (16.7 and 16.7%), nasopharyngitis (13.3 and 10.4%), fatigue (10 and 14.2%), headache (10 and 15.8%), and nausea (6.7 vs 11.4%) were frequent non-hematologic adverse events, whereas hypophosphatemia (23.3 and 6.8%), anemia (10 and 6.5%), and thrombocytopenia (3.3 and 10.2%) were the common hematologic/biochemical laboratory events.
Conclusion: Based on similar molecular response and safety results in both subgroups, we conclude that Ph-/BCR-ABL1 + patients benefit from nilotinib in the same way as Ph+ patients.
Keywords: Chronic myeloid leukemia; ENEST1st; Nilotinib; Philadelphia chromosome negative/BCR-ABL positive.
Conflict of interest statement
Figures


Similar articles
-
Rates of deep molecular response by digital and conventional PCR with frontline nilotinib in newly diagnosed chronic myeloid leukemia: a landmark analysis.Leuk Lymphoma. 2019 Oct;60(10):2384-2393. doi: 10.1080/10428194.2019.1590569. Epub 2019 Mar 26. Leuk Lymphoma. 2019. PMID: 30912699
-
Efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors.Haematologica. 2014 Jul;99(7):1191-6. doi: 10.3324/haematol.2013.096776. Epub 2014 Apr 4. Haematologica. 2014. PMID: 24705186 Free PMC article. Clinical Trial.
-
Impact of additional chromosomal aberrations and BCR-ABL kinase domain mutations on the response to nilotinib in Philadelphia chromosome-positive chronic myeloid leukemia.Haematologica. 2010 Apr;95(4):582-8. doi: 10.3324/haematol.2009.014712. Epub 2009 Dec 16. Haematologica. 2010. PMID: 20015884 Free PMC article.
-
Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia.Clin Ther. 2008 Nov;30(11):1956-75. doi: 10.1016/j.clinthera.2008.11.014. Clin Ther. 2008. PMID: 19108785 Review.
-
A rare BCR-ABL1 transcript in Philadelphia-positive acute myeloid leukemia: case report and literature review.BMC Cancer. 2019 Jan 10;19(1):50. doi: 10.1186/s12885-019-5265-5. BMC Cancer. 2019. PMID: 30630459 Free PMC article. Review.
Cited by
-
Prognostic significance of a normal karyotype in adult patients with BCR-ABL1-positive acute lymphoblastic leukemia in the tyrosine kinase inhibitor era.Clinics (Sao Paulo). 2020 Nov 11;75:e2011. doi: 10.6061/clinics/2020/e2011. eCollection 2020. Clinics (Sao Paulo). 2020. PMID: 33206758 Free PMC article.
-
Hematological Adverse Events with Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia: A Systematic Review with Meta-Analysis.Cancers (Basel). 2023 Aug 31;15(17):4354. doi: 10.3390/cancers15174354. Cancers (Basel). 2023. PMID: 37686630 Free PMC article. Review.
-
[Comparative study of molecular response of first-line and second-line nilotinib in patients with chronic-phase chronic myelogenous leukemia].Zhonghua Xue Ye Xue Za Zhi. 2019 Jun 14;40(6):522-525. doi: 10.3760/cma.j.issn.0253-2727.2019.06.014. Zhonghua Xue Ye Xue Za Zhi. 2019. PMID: 31340628 Free PMC article. Chinese. No abstract available.
-
Adult Ph-positive acute lymphoblastic leukemia-current concepts in cytogenetic abnormalities and outcomes.Am J Cancer Res. 2020 Aug 1;10(8):2309-2318. eCollection 2020. Am J Cancer Res. 2020. PMID: 32905489 Free PMC article. Review.
-
18 months follow-up of deep molecular response 4.5 (MR4.5) with nilotinib in patients with newly diagnosed chronic-phase chronic myeloid leukemia: a prospective, multi-center study in China.Front Med (Lausanne). 2023 Nov 16;10:1267512. doi: 10.3389/fmed.2023.1267512. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38034530 Free PMC article.
References
-
- Bartram CR et al (1983) Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature 306:277–280 - PubMed
-
- Bisen A, Claxton DF (2013) Tyrosine kinase targeted treatment of chronic myelogenous leukemia and other myeloproliferative neoplasms. Adv Exp Med Biol 779:179–196. doi:10.1007/978-1-4614-6176-0_8 - PubMed
-
- Cortes JE, Talpaz M, Beran M, O’Brien SM, Rios MB, Stass S, Kantarjian HM (1995) Philadelphia chromosome-negative chronic myelogenous leukemia with rearrangement of the breakpoint cluster region. Long-term follow-up results. Cancer 75:464–470 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

