Androgen Receptor Splice Variants Are Not Substrates of Nonsense-Mediated Decay
- PMID: 28224650
- PMCID: PMC5400682
- DOI: 10.1002/pros.23323
Androgen Receptor Splice Variants Are Not Substrates of Nonsense-Mediated Decay
Abstract
Background: Androgen receptor (AR) splice variants have been clinically associated with progressive cancer, castration-resistance, and resistance to AR antagonists and androgen synthesis inhibitors. AR variants can be generated by genomic alterations and alternative splicing, and their expression is androgen-regulated. There has been a suggestion that AR variants bearing premature termination codons and coding for truncated proteins should be regulated by the nonsense-mediated decay (NMD) mRNA surveillance pathway, suggesting that either the NMD pathway is dysfunctional in variant-expressing cell lines or that variants are somehow able to evade degradation by NMD.
Methods: We first used siRNA knockdown of the NMD regulator, UPF1, in an NMD reporter assay to determine if this surveillance pathway is functioning normally in AR variant-expressing cell lines. We then used UPF1 knockdown to determine if expression of the AR variants ARV3 and ARV7 is affected by inhibition of NMD. Next, we analyzed androgen regulation of UPF1 and used transcript expression analysis to determine if there is any association between UPF1 expression, resistance, and ARV3 or ARV7 expression.
Results: We found that the NMD pathway functions normally in the AR variant-expressing cell line 22Rv1 and that inhibition of NMD does not increase expression of ARV3 or ARV7. Furthermore, we found that expression of UPF1 is not androgen-regulated. We also found that UFP1 expression levels do not differentiate castration-sensitive from resistant cell line and that UPF1 expression does not correlate with expression of ARV3 or ARV7 in cells in which these variants are highly expressed.
Conclusion: This study eliminates a possible mechanism of regulation of certain AR variants. Future research into the regulation of AR variants should focus on other mechanisms to better understand the origin of these variants and to possibly inhibit their expression for the resensitization of resistant cancers. Prostate 77:829-837, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: UPF1; androgen receptor; nonsense-mediated decay; prostate cancer; splice variants.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

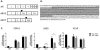
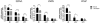

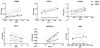
References
-
- Centenera MM, Harris JM, Tilley WD, Butler LM. Minireview: The Contribution of Different Androgen Receptor Domains to Receptor Dimerization and Signaling. Molecular Endocrinology. 2008;22(11):2373–2382. - PubMed
-
- Clegg N, Nelson PS. Androgen-Regulated Genes in the Prostate. In: Mohler J, Tindall D, editors. Androgen Action in Prostate Cancer. New York, NY: Springer US; 2009. pp. 631–661.
-
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. New England Journal of Medicine. 2014;371(5):424–433. - PMC - PubMed
-
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PFA, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE on behalf of the COUAAI. Randomized Phase 3 Trial of Abiraterone Acetate in Men with Metastatic Castration-Resistant Prostate Cancer and No Prior Chemotherapy. The New England journal of medicine. 2013;368(2):138–148. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

