Post-endocytic sorting of Plexin-D1 controls signal transduction and development of axonal and vascular circuits
- PMID: 28224988
- PMCID: PMC5322531
- DOI: 10.1038/ncomms14508
Post-endocytic sorting of Plexin-D1 controls signal transduction and development of axonal and vascular circuits
Abstract
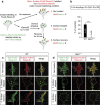
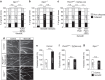
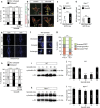
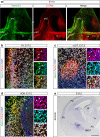
Local endocytic events involving receptors for axon guidance cues play a central role in controlling growth cone behaviour. Yet, little is known about the fate of internalized receptors, and whether the sorting events directing them to distinct endosomal pathways control guidance decisions. Here, we show that the receptor Plexin-D1 contains a sorting motif that interacts with the adaptor protein GIPC1 to facilitate transport to recycling endosomes. This sorting process promotes colocalization of Plexin-D1 with vesicular pools of active R-ras, leading to its inactivation. In the absence of interaction with GIPC1, missorting of Plexin-D1 results in loss of signalling activity. Consequently, Gipc1 mutant mice show specific defects in axonal projections, as well as vascular structures, that rely on Plexin-D1 signalling for their development. Thus, intracellular sorting steps that occur after receptor internalization by endocytosis provide a critical level of control of cellular responses to guidance signals.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
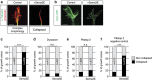
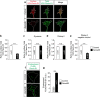
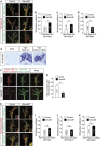
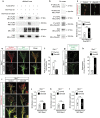

References
-
- Itofusa R. & Kamiguchi H. Polarizing membrane dynamics and adhesion for growth cone navigation. Mol. Cell. Neurosci. 48, 332–338 (2011). - PubMed
-
- Pfenninger K. H. Plasma membrane expansion: a neuron's Herculean task. Nat. Rev. Neurosci. 10, 251–261 (2009). - PubMed
-
- Zylbersztejn K. & Galli T. [Membrane traffic, a new actor in axon guidance]. Med. Sci. 28, 267–269 (2012). - PubMed
-
- Tojima T. & Kamiguchi H. Exocytic and endocytic membrane trafficking in axon development. Dev. Growth Differ. 57, 291–304 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

