UVRAG Deficiency Exacerbates Doxorubicin-Induced Cardiotoxicity
- PMID: 28225086
- PMCID: PMC5320807
- DOI: 10.1038/srep43251
UVRAG Deficiency Exacerbates Doxorubicin-Induced Cardiotoxicity
Abstract
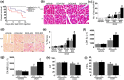
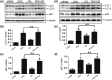
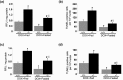
Doxorubicin (DOX) is an effective chemotherapeutic drug in the treatment of various types of cancers. However, its clinical application has been largely limited by potential development of cardiotoxicity. Previously we have shown that ultra-violet radiation resistance-associated gene (UVRAG), an autophagy-related protein, is essential for the maintenance of autophagic flux in the heart under physiological conditions. Here, we sought to determine the role of UVRAG-mediated autophagy in DOX-induced cardiotoxicity. Mouse models of acute or chronic DOX-induced cardiotoxicity were established. UVRAG deficiency exacerbated DOX-induced mortality and cardiotoxicity manifested by increased cytoplasmic vacuolization, enhanced collagen accumulation, elevated serum activities of lactate dehydrogenase and myocardial muscle creatine kinase, higher ROS levels, aggravated apoptosis and more depressed cardiac function. Autophagic flux was impaired in DOX-induced cardiotoxicity. UVRAG deficiency aggravated impaired autophagic flux in DOX-induced cardiotoxicity. Intermittent fasting restored autophagy and ameliorated pathological alterations of DOX-induced cardiotoxicity. Collectively, our data suggest that UVRAG deficiency exacerbates DOX-induced cardiotoxicity, at least in part, through aggravation of DOX-induced impaired autophagic flux. Intermittent fasting, which restores blunted autophagic flux and ameliorates pathology in the mouse models of DOX-induced cardiotoxicity, may be used as a potential preventive or therapeutic approach for DOX cardiotoxicity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Singal P. K. & Iliskovic N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 339, 900–905 (1998). - PubMed
-
- Takemura G. & Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 49, 330–352 (2007). - PubMed
-
- Octavia Y. et al.. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 52, 1213–1225 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

