Divergent functions of the left and right central amygdala in visceral nociception
- PMID: 28225716
- PMCID: PMC5655992
- DOI: 10.1097/j.pain.0000000000000830
Divergent functions of the left and right central amygdala in visceral nociception
Abstract
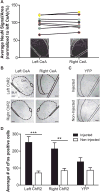
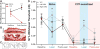
The left and right central amygdalae (CeA) are limbic regions involved in somatic and visceral pain processing. These 2 nuclei are asymmetrically involved in somatic pain modulation; pain-like responses on both sides of the body are preferentially driven by the right CeA, and in a reciprocal fashion, nociceptive somatic stimuli on both sides of the body predominantly alter molecular and physiological activities in the right CeA. Unknown, however, is whether this lateralization also exists in visceral pain processing and furthermore what function the left CeA has in modulating nociceptive information. Using urinary bladder distension (UBD) and excitatory optogenetics, a pronociceptive function of the right CeA was demonstrated in mice. Channelrhodopsin-2-mediated activation of the right CeA increased visceromotor responses (VMRs), while activation of the left CeA had no effect. Similarly, UBD-evoked VMRs increased after unilateral infusion of pituitary adenylate cyclase-activating polypeptide in the right CeA. To determine intrinsic left CeA involvement in bladder pain modulation, this region was optogenetically silenced during noxious UBD. Halorhodopsin (NpHR)-mediated inhibition of the left CeA increased VMRs, suggesting an ongoing antinociceptive function for this region. Finally, divergent left and right CeA functions were evaluated during abdominal mechanosensory testing. In naive animals, channelrhodopsin-2-mediated activation of the right CeA induced mechanical allodynia, and after cyclophosphamide-induced bladder sensitization, activation of the left CeA reversed referred bladder pain-like behaviors. Overall, these data provide evidence for functional brain lateralization in the absence of peripheral anatomical asymmetries.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




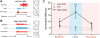
References
-
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. - PubMed
-
- Bauer RH. Lateralization of neural control for vocalization by the frog (Rana pipiens) Psychobiology. 1993;21:243–8.
-
- Bon K, Lanteri-Minet M, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: a c-fos and Krox-24 study at telencephalic levels, with a note on pituitary adenylate cyclase activating polypeptide (PACAP) Exp Brain Res. 1998;122:165–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

