Prefrontal cortex output circuits guide reward seeking through divergent cue encoding
- PMID: 28225752
- PMCID: PMC5772935
- DOI: 10.1038/nature21376
Prefrontal cortex output circuits guide reward seeking through divergent cue encoding
Abstract
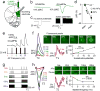
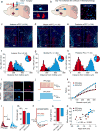
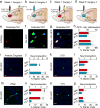
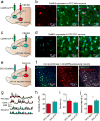
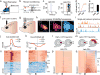
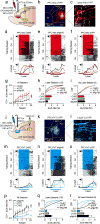
The prefrontal cortex is a critical neuroanatomical hub for controlling motivated behaviours across mammalian species. In addition to intra-cortical connectivity, prefrontal projection neurons innervate subcortical structures that contribute to reward-seeking behaviours, such as the ventral striatum and midline thalamus. While connectivity among these structures contributes to appetitive behaviours, how projection-specific prefrontal neurons encode reward-relevant information to guide reward seeking is unknown. Here we use in vivo two-photon calcium imaging to monitor the activity of dorsomedial prefrontal neurons in mice during an appetitive Pavlovian conditioning task. At the population level, these neurons display diverse activity patterns during the presentation of reward-predictive cues. However, recordings from prefrontal neurons with resolved projection targets reveal that individual corticostriatal neurons show response tuning to reward-predictive cues, such that excitatory cue responses are amplified across learning. By contrast, corticothalamic neurons gradually develop new, primarily inhibitory responses to reward-predictive cues across learning. Furthermore, bidirectional optogenetic manipulation of these neurons reveals that stimulation of corticostriatal neurons promotes conditioned reward-seeking behaviour after learning, while activity in corticothalamic neurons suppresses both the acquisition and expression of conditioned reward seeking. These data show how prefrontal circuitry can dynamically control reward-seeking behaviour through the opposing activities of projection-specific cell populations.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Langworthy OR. Increased spontaneous activity produced by frontal lobe lesion in cats. Am. J. Physiol. 1939;126:158–161.
-
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. - PubMed
-
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

