Elucidation of the biosynthesis of the methane catalyst coenzyme F430
- PMID: 28225763
- PMCID: PMC5337119
- DOI: 10.1038/nature21427
Elucidation of the biosynthesis of the methane catalyst coenzyme F430
Erratum in
-
Corrigendum: Elucidation of the biosynthesis of the methane catalyst coenzyme F430.Nature. 2017 May 3;545(7652):116. doi: 10.1038/nature22317. Nature. 2017. PMID: 28470207 No abstract available.
Abstract
Methane biogenesis in methanogens is mediated by methyl-coenzyme M reductase, an enzyme that is also responsible for the utilization of methane through anaerobic methane oxidation. The enzyme uses an ancillary factor called coenzyme F430, a nickel-containing modified tetrapyrrole that promotes catalysis through a methyl radical/Ni(ii)-thiolate intermediate. However, it is unclear how coenzyme F430 is synthesized from the common primogenitor uroporphyrinogen iii, incorporating 11 steric centres into the macrocycle, although the pathway must involve chelation, amidation, macrocyclic ring reduction, lactamization and carbocyclic ring formation. Here we identify the proteins that catalyse the biosynthesis of coenzyme F430 from sirohydrochlorin, termed CfbA-CfbE, and demonstrate their activity. The research completes our understanding of how the repertoire of tetrapyrrole-based pigments are constructed, permitting the development of recombinant systems to use these metalloprosthetic groups more widely.
Conflict of interest statement
The authors have no competing financial interests with this research.
Figures
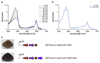

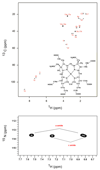
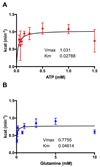


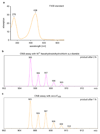

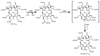


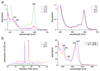
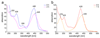
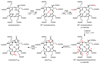
Comment in
-
Biochemistry: Origin of a key player in methane biosynthesis.Nature. 2017 Mar 2;543(7643):49-50. doi: 10.1038/nature21507. Epub 2017 Feb 22. Nature. 2017. PMID: 28225758 No abstract available.
References
-
- Ragsdale SW. Biochemistry of methyl-coenzyme M reductase: the nickel metalloenzyme that catalyzes the final step in synthesis and the first step in anaerobic oxidation of the greenhouse gas methane. Met Ions Life Sci. 2014;14:125–145. - PubMed
-
- Thauer RK. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology. 1998;144:2377–2406. - PubMed
-
- Raghoebarsing AA, et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature. 2006;440:918–921. - PubMed
-
- Scheller S, Goenrich M, Boecher R, Thauer RK, Jaun B. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature. 2010;465:606–608. - PubMed
-
- Shima S, et al. Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically. Nature. 2012;481:98–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

