Beyond Ketamine: New Approaches to the Development of Safer Antidepressants
- PMID: 28228087
- PMCID: PMC5652016
- DOI: 10.2174/1570159X15666170221101054
Beyond Ketamine: New Approaches to the Development of Safer Antidepressants
Abstract
Background: Ketamine has been reported to exert rapid and sustained antidepressant effects in patients with depression, including patients with treatment-resistant depression. However, ketamine has several drawbacks such as psychotomimetic/dissociative symptoms, abuse potential and neurotoxicity, all of which prevent its routine use in daily clinical practice.
Methods: Therefore, development of novel agents with fewer safety and usage concerns for the treatment of depression has been actively investigated. From this standpoint, searching for active substances (stereoisomers and metabolites) and agents acting on the N-methyl-D-aspartate (NMDA) receptor have recently gained much attention.
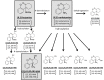
Results: The first approach includes stereoisomers of ketamine, (R)-ketamine and (S)-ketamine. Although (S)-ketamine has been considered as the active stereoisomer of racemic ketamine, recently, (R)-ketamine has been demonstrated to exert even more prolonged antidepressant effects in animal models than (S)-ketamine. Moreover, ketamine is rapidly metabolized into several metabolites, and some metabolites are speculated as being active substances exerting antidepressant effects. Of such metabolites, one in particular, namely, (2R,6R)-hydroxynorketamine, has been reported to be responsible for the antidepressant effects of ketamine. The second approach includes agents acting on the NMDA receptor, such as glycine site modulators and GluN2B subunit-selective antagonists. These agents have been tested in patients with treatment-resistant depression, and have been found to exhibit rapid antidepressant effects like ketamine.
Conclusion: The above approaches may be useful to overcome the drawbacks of ketamine. Elucidation of the mechanisms of action of ketamine may pave the way for the development of antidepressant that are safer, but as potent and rapidly acting as ketamine.
Keywords: (R)-ketamine; (S)-ketamine; 7-chlorokinurenic acid; GLYX-13; GluN2B; Ketamine; hydroxynorketamine; mGlu receptor..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures




References
-
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. [http://dx.doi.org/ 10.1001/archpsyc.62.6.593]. [PMID: 15939837]. - PubMed
-
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163(11):1905–1917. [http:// dx.doi.org/10.1176/ajp.2006.163.11.1905]. [PMID: 17074942]. - PubMed
-
- Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., Shores-Wilson K., Biggs M.M., Balasubramani G.K., Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006;163(1):28–40. [http://dx.doi.org/10.1176/appi.ajp.163.1.28]. [PMID: 16390886]. - PubMed
-
- Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7(5):426–437. [http://dx.doi.org/10.1038/nrd2462]. [PMID: 18425072]. - PMC - PubMed
-
- Skolnick P., Popik P., Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30(11):563–569. [http://dx.doi.org/10.1016/j.tips.2009.09.002]. [PMID: 19837463]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
