Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate
- PMID: 28228147
- PMCID: PMC5322615
- DOI: 10.1186/s12934-017-0648-2
Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate
Abstract
Background: Concerns regarding the safety of inactivated foot-and-mouth disease (FMD) vaccine have been raised since it is produced from cultured live FMD virus (FMDV). To overcome this issue, recombinant protein has been studied as an alternative vaccine.
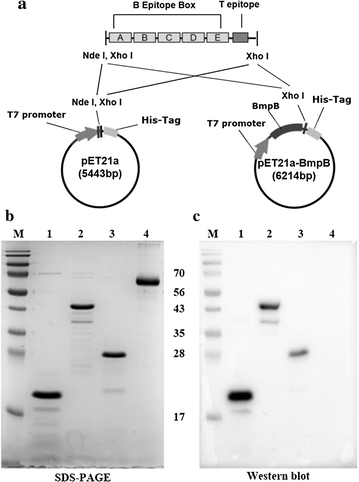
Results and conclusion: We designed a chimerical multi-epitope recombinant protein (5BT), which is comprised of tandem repeats of five B cell epitopes (residue of VP1 136-162) derived from different FMDV variants and one T-cell epitope (residue of 3A 21-35). To increase solubility and stability of 5BT, it was conjugated with BmpB, the membrane protein B of Brachyspira hyodysenteriae (B5BT). Our results indicated that 5BT was susceptible to degradation by host protease and produced with substantial fraction of inclusion body. The stability and solubility of 5BT was greatly increased by conjugating to BmpB. FMDV specific antibodies were observed in the serum of mice immunized with 5BT and B5BT comparable to inactivated FMD vaccine. Sera from 5BT and B5BT groups also exhibited high epitope-specific antibody titers in peptide specific ELISA, indicating that all five epitopes are exposed to the B cell receptor for the antibody reaction. Thus the multi-epitope recombinant protein designed in this study may be a potential candidate as an alternative vaccine against FMDV epidemic variants.
Keywords: Artificial recombinant protein; B cell epitope; FMDV; GH loop; Multi- epitope.
Figures




References
-
- Lee S-Y, Park M-E, Kim R-H, Ko M-K, Lee K-N, Kim S-M, Shim H-S, Kim B, Lee J-S, Park J-H. Genetic and immunologic relationships between vaccine and field strains for vaccine selection of type A foot-and-mouth disease virus circulating in East Asia. Vaccine. 2015;33:664–669. doi: 10.1016/j.vaccine.2014.12.007. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

