Cell Wall Remodeling in Abscission Zone Cells during Ethylene-Promoted Fruit Abscission in Citrus
- PMID: 28228766
- PMCID: PMC5296326
- DOI: 10.3389/fpls.2017.00126
Cell Wall Remodeling in Abscission Zone Cells during Ethylene-Promoted Fruit Abscission in Citrus
Erratum in
-
Corrigendum: Cell Wall Remodeling in Abscission Zone Cells during Ethylene-Promoted Fruit Abscission in Citrus.Front Plant Sci. 2017 Mar 7;8:301. doi: 10.3389/fpls.2017.00301. eCollection 2017. Front Plant Sci. 2017. PMID: 28275382 Free PMC article.
Abstract
Abscission is a cell separation process by which plants can shed organs such as fruits, leaves, or flowers. The process takes place in specific locations termed abscission zones. In fruit crops like citrus, fruit abscission represents a high percentage of annual yield losses. Thus, understanding the molecular regulation of abscission is of capital relevance to control production. To identify genes preferentially expressed within the citrus fruit abscission zone (AZ-C), we performed a comparative transcriptomics assay at the cell type resolution level between the AZ-C and adjacent fruit rind cells (non-abscising tissue) during ethylene-promoted abscission. Our strategy combined laser microdissection with microarray analysis. Cell wall modification-related gene families displayed prominent representation in the AZ-C. Phylogenetic analyses of such gene families revealed a link between phylogenetic proximity and expression pattern during abscission suggesting highly conserved roles for specific members of these families in abscission. Our transcriptomic data was validated with (and strongly supported by) a parallel approach consisting on anatomical, histochemical and biochemical analyses on the AZ-C during fruit abscission. Our work identifies genes potentially involved in organ abscission and provides relevant data for future biotechnology approaches aimed at controlling such crucial process for citrus yield.
Keywords: calyx abscission zone; cell wall modification; citrus fruit abscission; ethylene; lignin biosynthesis; phylogeny; transcriptomics.
Figures
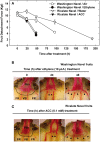
 , lignin deposition (phloroglucinol); FR, fruit rind; FD, floral disc; SP, sepals; VB, vascular bundles; P, parenchyma.
, lignin deposition (phloroglucinol); FR, fruit rind; FD, floral disc; SP, sepals; VB, vascular bundles; P, parenchyma.
 , separation line inside the AZ-C; CB, calyx button; FD, floral disc; FR, fruit rind; SP, sepal; VB, vascular bundles. Scale bars: 1 mm (A–E), 500 μm (A–C), 200 μm (E), 100 μm (C).
, separation line inside the AZ-C; CB, calyx button; FD, floral disc; FR, fruit rind; SP, sepal; VB, vascular bundles. Scale bars: 1 mm (A–E), 500 μm (A–C), 200 μm (E), 100 μm (C).
 , recently divided cell;
, recently divided cell;  , cell containing amyloplasts. Scale bars: 500 μm and 50 μm.
, cell containing amyloplasts. Scale bars: 500 μm and 50 μm.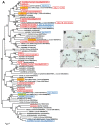
 ). AZ-C, abscission zone C; FR, fruit rind. Scale bars: 500 μm.
). AZ-C, abscission zone C; FR, fruit rind. Scale bars: 500 μm.
 ). AZ-C, abscission zone C; FR, fruit rind. Scale bars: 500 μm.
). AZ-C, abscission zone C; FR, fruit rind. Scale bars: 500 μm.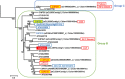

 ), 24 (
), 24 ( ) or 48 (
) or 48 ( ) h of ACC treatment. Micrographs represent the merger of images from pectic epitopes detection by mAbs (green) and from cellulose detection by calcofluor white (blue).
) h of ACC treatment. Micrographs represent the merger of images from pectic epitopes detection by mAbs (green) and from cellulose detection by calcofluor white (blue).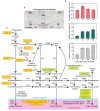

Similar articles
-
Comparative transcriptional survey between laser-microdissected cells from laminar abscission zone and petiolar cortical tissue during ethylene-promoted abscission in citrus leaves.BMC Plant Biol. 2009 Oct 23;9:127. doi: 10.1186/1471-2229-9-127. BMC Plant Biol. 2009. PMID: 19852773 Free PMC article.
-
Profiling gene expression in citrus fruit calyx abscission zone (AZ-C) treated with ethylene.Mol Genet Genomics. 2015 Oct;290(5):1991-2006. doi: 10.1007/s00438-015-1054-2. Epub 2015 May 7. Mol Genet Genomics. 2015. PMID: 25948248
-
Corrigendum: Cell Wall Remodeling in Abscission Zone Cells during Ethylene-Promoted Fruit Abscission in Citrus.Front Plant Sci. 2017 Mar 7;8:301. doi: 10.3389/fpls.2017.00301. eCollection 2017. Front Plant Sci. 2017. PMID: 28275382 Free PMC article.
-
Control of Organ Abscission and Other Cell Separation Processes by Evolutionary Conserved Peptide Signaling.Plants (Basel). 2019 Jul 15;8(7):225. doi: 10.3390/plants8070225. Plants (Basel). 2019. PMID: 31311120 Free PMC article. Review.
-
Development and regulation of pedicel abscission in tomato.Front Plant Sci. 2015 Jun 11;6:442. doi: 10.3389/fpls.2015.00442. eCollection 2015. Front Plant Sci. 2015. PMID: 26124769 Free PMC article. Review.
Cited by
-
Transcriptome analysis unravels key pathways and hub genes related to immature fruit abscission in Camellia oleifera.Front Plant Sci. 2024 Aug 9;15:1418358. doi: 10.3389/fpls.2024.1418358. eCollection 2024. Front Plant Sci. 2024. PMID: 39184578 Free PMC article.
-
Genome-wide analysis of AGO, DCL and RDR gene families reveals RNA-directed DNA methylation is involved in fruit abscission in Citrus sinensis.BMC Plant Biol. 2019 Sep 12;19(1):401. doi: 10.1186/s12870-019-1998-1. BMC Plant Biol. 2019. PMID: 31510935 Free PMC article.
-
Comparative transcriptomics of wild and commercial Citrus during early ripening reveals how domestication shaped fruit gene expression.BMC Plant Biol. 2022 Mar 17;22(1):123. doi: 10.1186/s12870-022-03509-9. BMC Plant Biol. 2022. PMID: 35300613 Free PMC article.
-
Abscission zone metabolism impacts pre- and post-harvest fruit quality: a very attaching story.Front Plant Sci. 2025 Jan 27;15:1524893. doi: 10.3389/fpls.2024.1524893. eCollection 2024. Front Plant Sci. 2025. PMID: 39980759 Free PMC article. Review.
-
Transcriptome analysis of the pulp of citrus fruitlets suggests that domestication enhanced growth processes and reduced chemical defenses increasing palatability.Front Plant Sci. 2022 Sep 2;13:982683. doi: 10.3389/fpls.2022.982683. eCollection 2022. Front Plant Sci. 2022. PMID: 36119632 Free PMC article.
References
-
- Addicot F. T. (1982). Abscission. University of California Press, Berkeley.
-
- Agustí J., Gimeno J., Merelo P., Serrano R., Cercos M., Conesa A., et al. . (2012). Early gene expression events in the laminar abscission zone of abscission-promoted citrus leaves after a cycle of water stress/rehydration: involvement of CitbHLH1. J. Exp. Bot. 63, 6079–6091. 10.1093/jxb/ers270 - DOI - PMC - PubMed
-
- Agustí J., Merelo P., Cercos M., Tadeo F. R., Talon M. (2009). Comparative transcriptional survey between laser-microdissected cells from laminar abscission zone and petiolar cortical tissue during ethylene-promoted abscission in citrus leaves. BMC Plant Biol. 9:127. 10.1186/1471-2229-9-127 - DOI - PMC - PubMed
-
- Argamasilla R., Gómez-Cadenas A., Arbona V. (2014). Metabolic and regulatory responses in citrus rootstocks in response to adverse environmental conditions. J. Plant Growth Regul. 33, 169–180. 10.1007/s00344-013-9359-z - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

