Vascularization and innervation of slits within polydimethylsiloxane sheets in the subcutaneous space of athymic nude mice
- PMID: 28228933
- PMCID: PMC5308423
- DOI: 10.1177/2041731417691645
Vascularization and innervation of slits within polydimethylsiloxane sheets in the subcutaneous space of athymic nude mice
Abstract
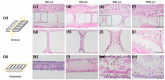
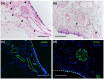
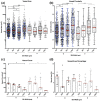
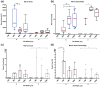
Success of cell therapy in avascular sites will depend on providing sufficient blood supply to transplanted tissues. A popular strategy of providing blood supply is to embed cells within a functionalized hydrogel implanted within the host to stimulate neovascularization. However, hydrogel systems are not always amenable for removal post-transplantation; thus, it may be advantageous to implant a device that contains cells while also providing access to the circulation so retrieval is possible. Here we investigate one instance of providing access to a vessel network, a thin sheet with through-cut slits, and determine if it can be vascularized from autologous materials. We compared the effect of slit width on vascularization of a thin sheet following subcutaneous implantation into an animal model. Polydimethylsiloxane sheets with varying slit widths (approximately 150, 300, 500, or 1500 µm) were fabricated from three-dimensional printed molds. Subcutaneous implantation of sheets in immunodeficient mice revealed that smaller slit widths have evidence of angiogenesis and new tissue growth, while larger slit widths contain native mature tissue squeezing into the space. Our results show that engineered slit sheets may provide a simple approach to cell transplantation by providing a prevascularized and innervated environment.
Keywords: Subcutaneous implant; innervation; micro-stereolithographic three-dimensional printing; polydimethylsiloxane; vascularization.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

