Involvement of Cellular Prion Protein in α-Synuclein Transport in Neurons
- PMID: 28229331
- PMCID: PMC5840251
- DOI: 10.1007/s12035-017-0451-4
Involvement of Cellular Prion Protein in α-Synuclein Transport in Neurons
Erratum in
-
Erratum to: Involvement of Cellular Prion Protein in α-Synuclein Transport in Neurons.Mol Neurobiol. 2018 Mar;55(3):1861. doi: 10.1007/s12035-017-0553-z. Mol Neurobiol. 2018. PMID: 28477141 Free PMC article. No abstract available.
Abstract
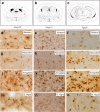
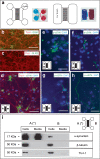
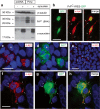
The cellular prion protein, encoded by the gene Prnp, has been reported to be a receptor of β-amyloid. Their interaction is mandatory for neurotoxic effects of β-amyloid oligomers. In this study, we aimed to explore whether the cellular prion protein participates in the spreading of α-synuclein. Results demonstrate that Prnp expression is not mandatory for α-synuclein spreading. However, although the pathological spreading of α-synuclein can take place in the absence of Prnp, α-synuclein expanded faster in PrPC-overexpressing mice. In addition, α-synuclein binds strongly on PrPC-expressing cells, suggesting a role in modulating the effect of α-synuclein fibrils.
Keywords: Amyloid spreading; Microfluidic devices; Prnp; Synuclein.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- BFU2015-67777-R/MINECO/International
- AGL2015-71764-REDT/MINECO/International
- DPI2015-64221-C2-1-R/MINECO/International
- BIO2015-63557-R/MINECO/International
- TEC2015-72718-EXP/MINECO/International
- SGR2014-1218/AGAUR/International
- 2015-FI-B-00817/AGAUR/International
- PI2014/02-4/Instituto de Salud Carlos III/International
- PRY-14-114/Instituto de Salud Carlos III/International
- FIS PIE14/00034/Instituto de Salud Carlos III/International
- PI14/00757/Instituto de Salud Carlos III/International
- PI10/01171/Instituto de Salud Carlos III/International
- ERC-2012-StG 306751/Erciyes Üniversitesi (TR)/International
- 278486/Seventh Framework Programme of the European Commission/International
- 228685-2/Seventh Framework Programme of the European Commission/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

