A SAMHD1 mutation associated with Aicardi-Goutières syndrome uncouples the ability of SAMHD1 to restrict HIV-1 from its ability to downmodulate type I interferon in humans
- PMID: 28229507
- PMCID: PMC5738905
- DOI: 10.1002/humu.23201
A SAMHD1 mutation associated with Aicardi-Goutières syndrome uncouples the ability of SAMHD1 to restrict HIV-1 from its ability to downmodulate type I interferon in humans
Abstract
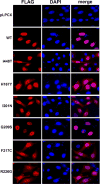
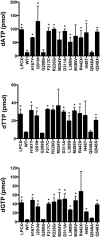
Mutations in the human SAMHD1 gene are known to correlate with the development of the Aicardi-Goutières syndrome (AGS), which is an inflammatory encephalopathy that exhibits neurological dysfunction characterized by increased production of type I interferon (IFN); this evidence has led to the concept that the SAMHD1 protein negatively regulates the type I IFN response. Additionally, the SAMHD1 protein has been shown to prevent efficient HIV-1 infection of macrophages, dendritic cells, and resting CD4+ T cells. To gain insights on the SAMHD1 molecular determinants that are responsible for the deregulated production of type I IFN, we explored the biochemical, cellular, and antiviral properties of human SAMHD1 mutants known to correlate with the development of AGS. Most of the studied SAMHD1 AGS mutants exhibit defects in the ability to oligomerize, decrease the levels of cellular deoxynucleotide triphosphates in human cells, localize exclusively to the nucleus, and restrict HIV-1 infection. At least half of the tested variants preserved the ability to be degraded by the lentiviral protein Vpx, and all of them interacted with RNA. Our investigations revealed that the SAMHD1 AGS variant p.G209S preserve all tested biochemical, cellular, and antiviral properties, suggesting that this residue is a determinant for the ability of SAMHD1 to negatively regulate the type I IFN response in human patients with AGS. Overall, our work genetically separated the ability of SAMHD1 to negatively regulate the type I IFN response from its ability to restrict HIV-1.
Keywords: Aicardi-Goutières syndrome; HIV-1; SAMHD1; interferon.
© 2017 Wiley Periodicals, Inc.
Figures


Similar articles
-
SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutières syndrome are highly susceptible to HIV-1 infection.PLoS Pathog. 2011 Dec;7(12):e1002425. doi: 10.1371/journal.ppat.1002425. Epub 2011 Dec 8. PLoS Pathog. 2011. PMID: 22174685 Free PMC article.
-
The ribonuclease activity of SAMHD1 is required for HIV-1 restriction.Nat Med. 2014 Aug;20(8):936-41. doi: 10.1038/nm.3626. Epub 2014 Jul 20. Nat Med. 2014. PMID: 25038827 Free PMC article.
-
SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells.Nat Med. 2012 Nov;18(11):1682-7. doi: 10.1038/nm.2964. Nat Med. 2012. PMID: 22972397 Free PMC article.
-
Roles of SAMHD1 in antiviral defense, autoimmunity and cancer.Rev Med Virol. 2017 Jul;27(4). doi: 10.1002/rmv.1931. Epub 2017 Apr 25. Rev Med Virol. 2017. PMID: 28444859 Review.
-
SAMHD1: Recurring roles in cell cycle, viral restriction, cancer, and innate immunity.Autoimmunity. 2018 May;51(3):96-110. doi: 10.1080/08916934.2018.1454912. Epub 2018 Mar 27. Autoimmunity. 2018. PMID: 29583030 Free PMC article. Review.
Cited by
-
SAMHD1 deficient human monocytes autonomously trigger type I interferon.Mol Immunol. 2018 Sep;101:450-460. doi: 10.1016/j.molimm.2018.08.005. Epub 2018 Aug 9. Mol Immunol. 2018. PMID: 30099227 Free PMC article.
-
Activation and Allostery in a Fungal SAMHD1 Hydrolase: An Evolutionary Blueprint for dNTP Catabolism.JACS Au. 2025 Apr 17;5(4):1862-1874. doi: 10.1021/jacsau.5c00090. eCollection 2025 Apr 28. JACS Au. 2025. PMID: 40313832 Free PMC article.
-
The SAMHD1 rs6029941 (A/G) Polymorphism Seems to Influence the HTLV-1 Proviral Load and IFN-Alpha Levels.Front Cell Infect Microbiol. 2020 May 25;10:246. doi: 10.3389/fcimb.2020.00246. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32656092 Free PMC article.
-
Structural and functional characterization explains loss of dNTPase activity of the cancer-specific R366C/H mutant SAMHD1 proteins.J Biol Chem. 2021 Oct;297(4):101170. doi: 10.1016/j.jbc.2021.101170. Epub 2021 Sep 4. J Biol Chem. 2021. PMID: 34492268 Free PMC article.
-
Are Evolution and the Intracellular Innate Immune System Key Determinants in HIV Transmission?Front Immunol. 2017 Oct 6;8:1246. doi: 10.3389/fimmu.2017.01246. eCollection 2017. Front Immunol. 2017. PMID: 29056936 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

