Regulated methionine oxidation by monooxygenases
- PMID: 28229915
- PMCID: PMC5540442
- DOI: 10.1016/j.freeradbiomed.2017.02.010
Regulated methionine oxidation by monooxygenases
Abstract
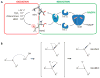
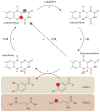
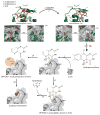
Protein function can be regulated via post-translational modifications by numerous enzymatic and non-enzymatic mechanisms, including oxidation of cysteine and methionine residues. Redox-dependent regulatory mechanisms have been identified for nearly every cellular process, but the major paradigm has been that cellular components are oxidized (damaged) by reactive oxygen species (ROS) in a relatively unspecific way, and then reduced (repaired) by designated reductases. While this scheme may work with cysteine, it cannot be ascribed to other residues, such as methionine, whose reaction with ROS is too slow to be biologically relevant. However, methionine is clearly oxidized in vivo and enzymes for its stereoselective reduction are present in all three domains of life. Here, we revisit the chemistry and biology of methionine oxidation, with emphasis on its generation by enzymes from the monooxygenase family. Particular attention is placed on MICALs, a recently discovered family of proteins that harbor an unusual flavin-monooxygenase domain with an NADPH-dependent methionine sulfoxidase activity. Based on structural and kinetic information we provide a rational framework to explain MICAL mechanism, inhibition, and regulation. Methionine residues that are targeted by MICALs are reduced back by methionine sulfoxide reductases, suggesting that reversible methionine oxidation may be a general mechanism analogous to the regulation by phosphorylation by kinases/phosphatases. The identification of new enzymes that catalyze the oxidation of methionine will open a new area of research at the forefront of redox signaling.
Keywords: Actin; DUF3585; MICAL; Methionine; Methionine oxidation; Methionine sulfoxide; Methionine sulfoxide reductase; Monooxygenase; Multidomain proteins; Redox signaling; Scaffolding proteins.
Copyright © 2017. Published by Elsevier Inc.
Figures


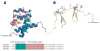


References
-
- Flohé L. Methods in Enzymology. 2010;473:1–39. - PubMed
-
- Rhee SG, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Current Opinion in Cell Biology. 2005;17:183–189. - PubMed
-
- Bak DW, et al. Cysteine-mediated redox signalling in the mitochondria. Mol Bio Syst. 2015;11:678–697. - PubMed
-
- Leichert LI, Dick TP. Incidence and physiological relevance of protein thiol switches. Biological Chemistry. 2015;396:389–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

