Gel phase formation in dilute triblock copolyelectrolyte complexes
- PMID: 28230046
- PMCID: PMC5331217
- DOI: 10.1038/ncomms14131
Gel phase formation in dilute triblock copolyelectrolyte complexes
Abstract
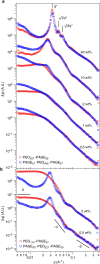
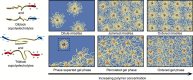
Assembly of oppositely charged triblock copolyelectrolytes into phase-separated gels at low polymer concentrations (<1% by mass) has been observed in scattering experiments and molecular dynamics simulations. Here we show that in contrast to uncharged, amphiphilic block copolymers that form discrete micelles at low concentrations and enter a phase of strongly interacting micelles in a gradual manner with increasing concentration, the formation of a dilute phase of individual micelles is prevented in polyelectrolyte complexation-driven assembly of triblock copolyelectrolytes. Gel phases form and phase separate almost instantaneously on solvation of the copolymers. Furthermore, molecular models of self-assembly demonstrate the presence of oligo-chain aggregates in early stages of copolyelectrolyte assembly, at experimentally unobservable polymer concentrations. Our discoveries contribute to the fundamental understanding of the structure and pathways of complexation-driven assemblies, and raise intriguing prospects for gel formation at extraordinarily low concentrations, with applications in tissue engineering, agriculture, water purification and theranostics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Structure of pH sensitive self-assembled amphiphilic di- and triblock copolyelectrolytes: micelles, aggregates and transient networks.Phys Chem Chem Phys. 2013 Mar 21;15(11):3955-64. doi: 10.1039/c3cp43653e. Phys Chem Chem Phys. 2013. PMID: 23389029
-
Formation of complexes in aqueous solutions of amphiphilic triblock polyelectrolytes of different topologies and an oppositely charged protein.Soft Matter. 2018 Apr 18;14(15):2860-2869. doi: 10.1039/C8SM00208H. Soft Matter. 2018. PMID: 29565433
-
Complex micelles from the self-assembly of amphiphilic triblock copolymers in selective solvents.J Chem Phys. 2010 May 28;132(20):204905. doi: 10.1063/1.3431203. J Chem Phys. 2010. PMID: 20515112
-
Responsive Hydrogels from Associative Block Copolymers: Physical Gelling through Polyion Complexation.Gels. 2017 Jan 1;3(1):3. doi: 10.3390/gels3010003. Gels. 2017. PMID: 30920500 Free PMC article. Review.
-
Gels Obtained by Colloidal Self-Assembly of Amphiphilic Molecules.Gels. 2017 Aug 3;3(3):30. doi: 10.3390/gels3030030. Gels. 2017. PMID: 30920526 Free PMC article. Review.
Cited by
-
A short peptide synthon for liquid-liquid phase separation.Nat Chem. 2021 Nov;13(11):1046-1054. doi: 10.1038/s41557-021-00788-x. Epub 2021 Oct 11. Nat Chem. 2021. PMID: 34645986
-
Form Equals Function: Influence of Coacervate Architecture on Drug Delivery Applications.ACS Biomater Sci Eng. 2024 Nov 11;10(11):6766-6789. doi: 10.1021/acsbiomaterials.4c01105. Epub 2024 Oct 18. ACS Biomater Sci Eng. 2024. PMID: 39423330 Free PMC article. Review.
-
Sequence and entropy-based control of complex coacervates.Nat Commun. 2017 Nov 2;8(1):1273. doi: 10.1038/s41467-017-01249-1. Nat Commun. 2017. PMID: 29097695 Free PMC article.
-
Relaxation Behavior by Time-Salt and Time-Temperature Superpositions of Polyelectrolyte Complexes from Coacervate to Precipitate.Gels. 2018 Jan 18;4(1):11. doi: 10.3390/gels4010011. Gels. 2018. PMID: 30674787 Free PMC article.
-
Comparing Zwitterionic and PEG Exteriors of Polyelectrolyte Complex Micelles.Molecules. 2020 May 30;25(11):2553. doi: 10.3390/molecules25112553. Molecules. 2020. PMID: 32486282 Free PMC article.
References
-
- Harada A. & Kataoka K. Chain length recognition: core–shell supramolecular assembly from oppositely charged block copolymers. Science 283, 65–67 (1999). - PubMed
-
- Voets I. K., de Keizer A. & Cohen Stuart M. A. Complex coacervate core micelles. Adv. Colloid Interface Sci. 147, 300–318 (2009). - PubMed
-
- Pergushov D. V., Mueller A. H. E. & Schacher F. H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 41, 6888–6901 (2012). - PubMed
-
- Ou Z. & Muthukumar M. Entropy and enthalpy of polyelectrolyte complexation: Langevin dynamics simulations. J. Chem. Phys. 124, 154902 (2006). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

