α-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson's disease patients
- PMID: 28230073
- PMCID: PMC5322400
- DOI: 10.1038/srep42984
α-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson's disease patients
Abstract
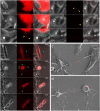
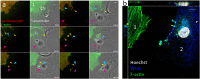
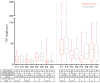
Parkinson's disease (PD) is characterized by the presence of inclusions known as Lewy bodies, which mainly consist of α-synuclein (α-syn) aggregates. There is growing evidence that α-syn self-propagates in non-neuronal cells, thereby contributing to the progression and spread of PD pathology in the brain. Tunneling nanotubes (TNTs) are long, thin, F-actin-based membranous channels that connect cells and have been proposed to act as conduits for α-syn transfer between cells. SH-SY5Y cells and primary human brain pericytes, derived from postmortem PD brains, frequently form TNTs that allow α-syn transfer and long-distance electrical coupling between cells. Pericytes in situ contain α-syn precipitates like those seen in neurons. Exchange through TNTs was rapid, but dependent on the size of the protein. Proteins were able to spread throughout a network of cells connected by TNTs. Transfer through TNTs was not restricted to α-syn; fluorescent control proteins and labeled membrane were also exchanged through TNTs. Most importantly the formation of TNTs and transfer continued during mitosis. Together, our results provide a detailed description of TNTs in SH-SY5Y cells and human brain PD pericytes, demonstrating their role in α-syn transfer and further emphasize the importance that non-neuronal cells, such as pericytes play in disease progression.
Figures



References
-
- Li J.-Y. et al.. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14, 501–503 (2008). - PubMed
-
- Kordower J. H., Chu Y., Hauser R. A., Freeman T. B. & Olanow C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14, 504–506 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

