Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients
- PMID: 28230269
- PMCID: PMC5510084
- DOI: 10.1111/bcp.13270
Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients
Abstract
Aims: Rituximab is a monoclonal antibody directed against CD20, which is approved in rheumatoid arthritis (RA). This study aimed at assessing the influence of CD19+ cell counts as target-antigen amount, and of immunoglobulin G (IgG) serum concentrations on rituximab pharmacokinetics in RA patients.
Methods: In a cohort of 64 RA patients who had received repetitive courses of rituximab, the influence of CD19+ cell count, IgG serum concentration, body surface area, sex and disease activity score in 28 joints on rituximab pharmacokinetic parameters was assessed using a population pharmacokinetic analysis.
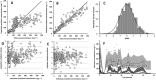
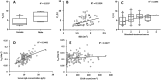
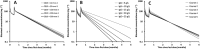
Results: A two-compartment model, with first-order distribution and elimination best described the data. The volume of distribution of central compartment and clearance of rituximab were estimated at 4.7 l and 0.56 l day-1 , respectively. Distribution and elimination half-lives were 0.9 days and 17.3 days, respectively. As expected, the central volume of distribution increased with body surface area (P = 0.012) and was higher in male than in female (P = 0.004). We found that the elimination rate constant (k10 ) increased with CD19+ count (P = 0.00022) and IgG concentration (P = 7.4 × 10-8 ), and that k10 decreased with time (P = 0.00015), partly explained by a change in target-antigen amount.
Conclusions: The association between CD19+ count and k10 may be explained by target-mediated drug disposition, while the association between IgG serum concentration and k10 may be explained by a saturation of the neonatal Fc receptor at high IgG concentrations, resulting in decreased recycling of rituximab.
Keywords: elimination rate constant; immunoglobulin; pharmacokinetics; rheumatoid arthritis; rituximab.
© 2017 The British Pharmacological Society.
Figures
References
-
- Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet 2010; 376: 1094–1108. - PubMed
-
- Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83: 435–445. - PubMed
-
- Pham T, Fautrel B, Gottenberg JE, Goupille P, Hachulla E, Masson C, et al. Rituximab (MabThera) therapy and safety management. Clinical tool guide. Joint Bone Spine 2008; 75: S1–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

