Comparative Effectiveness of Rapid-Acting Insulins in Adults with Diabetes
- PMID: 28230457
- PMCID: PMC10397578
- DOI: 10.18553/jmcp.2017.23.3.291
Comparative Effectiveness of Rapid-Acting Insulins in Adults with Diabetes
Abstract
Background: Although there are a variety of insulin products and new delivery modalities available, the absence of direct clinical and economic comparisons can make treatment planning and formulary decision making difficult. Direct comparisons between insulin aspart and insulin lispro from a large heterogeneous population are not available.
Objective: To assess differences in clinical outcomes, medication adherence, utilization, and total health care costs between aspart and lispro and vial versus pen modalities for administering these short-acting insulin analogs.
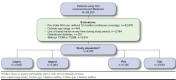
Methods: This retrospective cohort study used administrative claims data from the Humana Research Database to identify people with type 1 or type 2 diabetes and Medicare or commercial insurance (with medical and pharmacy benefits) who newly initiated rapid-acting insulin between January 1, 2008, and December 31, 2013, and were continuously enrolled during the 12-month baseline and 12-month follow-up periods. Generalized linear models were used to assess differences in costs and utilization. Logistic regression models measured the likelihood of having a hypoglycemic event, worsening diabetes complications, or a change in glycated hemoglobin (A1c).
Results: 8,189 patients included in the study were grouped by rapid-acting insulin product (aspart, n = 5,364, and lispro, n = 2,566) and modality (vial, n = 6,135, and pen, n = 2,054). There were no significant differences in the percentage of patients with a hypoglycemic event, new or worsening diabetes complications, or change in A1c, and there were no significant differences in adjusted total health care, medical and pharmacy costs, or emergency department visits between any of the product or modality comparisons. There was a significant difference in mean annual inpatient stays between lispro and aspart (adjusted mean = 2.24, 95% CI = 0.73-6.69, and adjusted mean = 2.65, 95% CI = 0.86-7.86, respectively; P < 0.001) and pen and vial cohorts (adjusted mean = 1.74, 95% CI = 0.56-4.99, and adjusted mean = 3.05, 95% CI = 1.01-9.08, respectively; P < 0.001). Adherence was similar for the lispro and aspart cohorts. Adherence was higher in the pen cohort (as measured by medication possession ratio ≥80%) compared with the vial cohort (adjusted odds ratio = 1.29, 95% CI = 1.12-1.50).
Conclusions: This study provides a comprehensive assessment of outcomes and costs between 2 commonly used rapid-acting insulin products. Overall, there was little differentiation between products, although adherence improved significantly with pen devices. These findings may simplify decisions related to formulary options and choice of therapy.
Disclosures: No outside funding supported this study. Racsa and Ellis are employees of Comprehensive Health Insights, a subsidiary of Humana, and Saverno was employed with Comprehensive Health Insights at the time of this study. Meah is an employee of, and owns stock in, Humana. The authors have no financial disclosures or potential conflicts of interest to report. All authors contributed equally to study concept and design, data interpretation, and manuscript preparation. Racsa collected the data.
Conflict of interest statement
No outside funding supported this study. Racsa and Ellis are employees of Comprehensive Health Insights, a subsidiary of Humana, and Saverno was employed with Comprehensive Health Insights at the time of this study. Meah is an employee of, and owns stock in, Humana. The authors have no financial disclosures or potential conflicts of interest to report.
All authors contributed equally to study concept and design, data interpretation, and manuscript preparation. Racsa collected the data.
References
-
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. .. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(1):84-113. - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. .. Management of hypergly-cemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-49. - PubMed
-
- Meece J. Dispelling myths and removing barriers about insulin in type 2 diabetes. Diabetes Educ. 2006;32(1 Suppl):S9-S18. - PubMed
-
- Petrak F, Stridde E, Leverkus F, Crispin AA, Forst T, Pfützner A.. Development and validation of a new measure to evaluate psychological resistance to insulin treatment. Diabetes Care. 2007;30(9):2199-204. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical