CVD and Oxidative Stress
- PMID: 28230726
- PMCID: PMC5332926
- DOI: 10.3390/jcm6020022
CVD and Oxidative Stress
Abstract
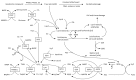
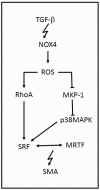
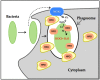
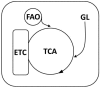
Nowadays, it is known that oxidative stress plays at least two roles within the cell, the generation of cellular damage and the involvement in several signaling pathways in its balanced normal state. So far, a substantial amount of time and effort has been expended in the search for a clear link between cardiovascular disease (CVD) and the effects of oxidative stress. Here, we present an overview of the different sources and types of reactive oxygen species in CVD, highlight the relationship between CVD and oxidative stress and discuss the most prominent molecules that play an important role in CVD pathophysiology. Details are given regarding common pharmacological treatments used for cardiovascular distress and how some of them are acting upon ROS-related pathways and molecules. Novel therapies, recently proposed ROS biomarkers, as well as future challenges in the field are addressed. It is apparent that the search for a better understanding of how ROS are contributing to the pathophysiology of CVD is far from over, and new approaches and more suitable biomarkers are needed for the latter to be accomplished.
Keywords: CVD; cardiovascular disease; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

