Metabolic Effects of Glucose-Fructose Co-Ingestion Compared to Glucose Alone during Exercise in Type 1 Diabetes
- PMID: 28230765
- PMCID: PMC5331595
- DOI: 10.3390/nu9020164
Metabolic Effects of Glucose-Fructose Co-Ingestion Compared to Glucose Alone during Exercise in Type 1 Diabetes
Abstract
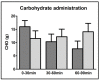
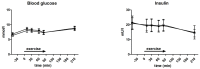
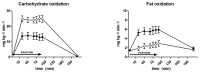
This paper aims to compare the metabolic effects of glucose-fructose co-ingestion (GLUFRU) with glucose alone (GLU) in exercising individuals with type 1 diabetes mellitus. Fifteen male individuals with type 1 diabetes (HbA1c 7.0% ± 0.6% (53 ± 7 mmol/mol)) underwent a 90 min iso-energetic continuous cycling session at 50% VO2max while ingesting combined glucose-fructose (GLUFRU) or glucose alone (GLU) to maintain stable glycaemia without insulin adjustment. GLUFRU and GLU were labelled with 13C-fructose and 13C-glucose, respectively. Metabolic assessments included measurements of hormones and metabolites, substrate oxidation, and stable isotopes. Exogenous carbohydrate requirements to maintain stable glycaemia were comparable between GLUFRU and GLU (p = 0.46). Fat oxidation was significantly higher (5.2 ± 0.2 vs. 2.6 ± 1.2 mg·kg-1·min-1, p < 0.001) and carbohydrate oxidation lower (18.1 ± 0.8 vs. 24.5 ± 0.8 mg·kg-1·min-1p < 0.001) in GLUFRU compared to GLU, with decreased muscle glycogen oxidation in GLUFRU (10.2 ± 0.9 vs. 17.5 ± 1.0 mg·kg-1·min-1, p < 0.001). Lactate levels were higher (2.2 ± 0.2 vs. 1.8 ± 0.1 mmol/L, p = 0.012) in GLUFRU, with comparable counter-regulatory hormones between GLUFRU and GLU (p > 0.05 for all). Glucose and insulin levels, and total glucose appearance and disappearance were comparable between interventions. Glucose-fructose co-ingestion may have a beneficial impact on fuel metabolism in exercising individuals with type 1 diabetes without insulin adjustment, by increasing fat oxidation whilst sparing glycogen.
Keywords: carbohydrates; exercise; fructose; glucose; glycaemia; substrate oxidation; type 1 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose.Am J Clin Nutr. 2010 Nov;92(5):1071-9. doi: 10.3945/ajcn.2010.29566. Epub 2010 Sep 8. Am J Clin Nutr. 2010. PMID: 20826630 Clinical Trial.
-
Fructose Coingestion Does Not Accelerate Postexercise Muscle Glycogen Repletion.Med Sci Sports Exerc. 2016 May;48(5):907-12. doi: 10.1249/MSS.0000000000000829. Med Sci Sports Exerc. 2016. PMID: 26606271 Clinical Trial.
-
Fructose and Sucrose Intake Increase Exogenous Carbohydrate Oxidation during Exercise.Nutrients. 2017 Feb 20;9(2):167. doi: 10.3390/nu9020167. Nutrients. 2017. PMID: 28230742 Free PMC article. Clinical Trial.
-
Glucose-fructose ingestion and exercise performance: The gastrointestinal tract and beyond.Eur J Sport Sci. 2017 Aug;17(7):874-884. doi: 10.1080/17461391.2017.1317035. Epub 2017 Apr 25. Eur J Sport Sci. 2017. PMID: 28441908 Review.
-
Glucose Plus Fructose Ingestion for Post-Exercise Recovery-Greater than the Sum of Its Parts?Nutrients. 2017 Mar 30;9(4):344. doi: 10.3390/nu9040344. Nutrients. 2017. PMID: 28358334 Free PMC article. Review.
Cited by
-
Is intake of fruit juice useful in exercise-induced hypoglycemia prevention in individuals with type 1 diabetes mellitus?Front Endocrinol (Lausanne). 2022 Oct 21;13:1045639. doi: 10.3389/fendo.2022.1045639. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36339413 Free PMC article. No abstract available.
-
Carbohydrate Intake in the Context of Exercise in People with Type 1 Diabetes.Nutrients. 2019 Dec 10;11(12):3017. doi: 10.3390/nu11123017. Nutrients. 2019. PMID: 31835538 Free PMC article. Review.
-
Recovery Phase Nutrition and Insulin Strategies for a Collegiate Distance Runner with Type 1 Diabetes Mellitus: A Case Study.Sports (Basel). 2023 Nov 3;11(11):214. doi: 10.3390/sports11110214. Sports (Basel). 2023. PMID: 37999431 Free PMC article.
-
Fructose, Glucocorticoids and Adipose Tissue: Implications for the Metabolic Syndrome.Nutrients. 2017 Apr 26;9(5):426. doi: 10.3390/nu9050426. Nutrients. 2017. PMID: 28445389 Free PMC article. Review.
-
A Sweet Connection? Fructose's Role in Hepatocellular Carcinoma.Biomolecules. 2020 Mar 25;10(4):496. doi: 10.3390/biom10040496. Biomolecules. 2020. PMID: 32218179 Free PMC article. Review.
References
-
- LaPorte R.E., Dorman J.S., Tajima N., Cruickshanks K.J., Orchard T.J., Cavender D.E., Becker D.J., Drash A.L. Pittsburgh Insulin-Dependent Diabetes Mellitus Morbidity and Mortality Study: Physical activity and diabetic complications. Pediatrics. 1986;78:1027–1033. - PubMed
-
- Zoppini G., Carlini M., Muggeo M. Self-reported exercise and quality of life in young type 1 diabetic subjects. Diabetes Nutr. Metab. 2003;16:77–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

