Characterization of Carbonic Anhydrase 9 in the Alimentary Canal of Aedes aegypti and Its Relationship to Homologous Mosquito Carbonic Anhydrases
- PMID: 28230813
- PMCID: PMC5334767
- DOI: 10.3390/ijerph14020213
Characterization of Carbonic Anhydrase 9 in the Alimentary Canal of Aedes aegypti and Its Relationship to Homologous Mosquito Carbonic Anhydrases
Abstract
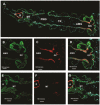
In the mosquito midgut, luminal pH regulation and cellular ion transport processes are important for the digestion of food and maintenance of cellular homeostasis. pH regulation in the mosquito gut is affected by the vectorial movement of the principal ions including bicarbonate/carbonate and protons. As in all metazoans, mosquitoes employ the product of aerobic metabolism carbon dioxide in its bicarbonate/carbonate form as one of the major buffers of cellular and extracellular pH. The conversion of metabolic carbon dioxide to bicarbonate/carbonate is accomplished by a family of enzymes encoded by the carbonic anhydrase gene family. This study characterizes Aedes aegypti carbonic anhydrases using bioinformatic, molecular, and immunohistochemical methods. Our analyses show that there are fourteen Aedes aegypti carbonic anhydrase genes, two of which are expressed as splice variants. The carbonic anhydrases were classified as either integral membrane, peripheral membrane, mitochondrial, secreted, or soluble cytoplasmic proteins. Using polymerase chain reaction and Western blotting, one of the carbonic anhydrases, Aedes aegypti carbonic anhydrase 9, was analyzed and found in each life stage, male/female pupae, male/female adults, and in the female posterior midgut. Next, carbonic anhydrase 9 was analyzed in larvae and adults using confocal microscopy and was detected in the midgut regions. According to our analyses, carbonic anhydrase 9 is a soluble cytoplasmic enzyme found in the alimentary canal of larvae and adults and is expressed throughout the life cycle of the mosquito. Based on previous physiological analyses of adults and larvae, it appears AeCA9 is one of the major carbonic anhydrases involved in producing bicarbonate/carbonate which is involved in pH regulation and ion transport processes in the alimentary canal. Detailed understanding of the molecular bases of ion homeostasis in mosquitoes will provide targets for novel mosquito control strategies into the new millennium.
Keywords: alkaline; carbonic anhydrase (CA); data mining; immunohistochemistry; midgut; molecular phylogenetics; mosquito; pH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Carbonic anhydrases and anion transport in mosquito midgut pH regulation.J Exp Biol. 2009 Jun;212(Pt 11):1662-71. doi: 10.1242/jeb.028084. J Exp Biol. 2009. PMID: 19448076 Free PMC article. Review.
-
Carbonic anhydrase in the midgut of larval Aedes aegypti: cloning, localization and inhibition.J Exp Biol. 2002 Mar;205(Pt 5):591-602. doi: 10.1242/jeb.205.5.591. J Exp Biol. 2002. PMID: 11907049
-
A GPI-linked carbonic anhydrase expressed in the larval mosquito midgut.J Exp Biol. 2004 Dec;207(Pt 26):4559-72. doi: 10.1242/jeb.01287. J Exp Biol. 2004. PMID: 15579552
-
Slc4-like anion transporters of the larval mosquito alimentary canal.J Insect Physiol. 2012 Apr;58(4):551-62. doi: 10.1016/j.jinsphys.2012.01.002. Epub 2012 Jan 11. J Insect Physiol. 2012. PMID: 22251674 Free PMC article.
-
The Fundamental Role of Bicarbonate Transporters and Associated Carbonic Anhydrase Enzymes in Maintaining Ion and pH Homeostasis in Non-Secretory Organs.Int J Mol Sci. 2020 Jan 4;21(1):339. doi: 10.3390/ijms21010339. Int J Mol Sci. 2020. PMID: 31947992 Free PMC article. Review.
Cited by
-
Unravelling the genome of the brackish water malaria vector Anopheles aquasalis.Sci Rep. 2023 Nov 22;13(1):20472. doi: 10.1038/s41598-023-47830-1. Sci Rep. 2023. PMID: 37993652 Free PMC article.
-
Larvicidal Activity of Cinnamic Acid Derivatives: Investigating Alternative Products for Aedes aegypti L. Control.Molecules. 2020 Dec 24;26(1):61. doi: 10.3390/molecules26010061. Molecules. 2020. PMID: 33374484 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

