Sudden versus gradual pressure wean from Nasal CPAP in preterm infants: a randomized controlled trial
- PMID: 28230835
- PMCID: PMC5446290
- DOI: 10.1038/jp.2017.10
Sudden versus gradual pressure wean from Nasal CPAP in preterm infants: a randomized controlled trial
Abstract
Objective: In preterm infants, nasal continuous positive airway pressure (NCPAP) is widely used for treatment of respiratory distress syndrome. However, the strategies for successfully weaning infants off NCPAP are still not well defined and there remains considerable variation between the methods. The objective of this study is to determine whether gradual weaning of NCPAP pressure is more successful than sudden weaning off NCPAP to room air.
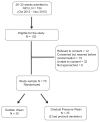
Study design: A randomized controlled trial was conducted in a level 3 neonatal intensive care unit on 70 preterm neonates who were born between 26 and 32 weeks gestation and required NCPAP for at least 48 h. When infants were stable on NCPAP at 0.21 FiO2 and 5 cm H2O positive end expiratory pressure, neonates were randomized to the gradual wean group (reduction in pressure by 1 cm every 8 h until 3 cm H20 was reached) or to sudden wean group (one time NCPAP removal to room air). The primary outcome was a success at the first trial to wean to room air. Secondary outcomes were a number of trials, and weight and postmenstrual age (PMA) at the time of successful wean. Total number of days on NCPAP and length of stay (LOS) in the hospital were also compared between the groups.
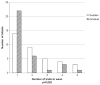
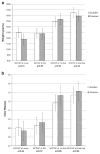
Results: Of the 70 infants included in the study, 35 were randomized to sudden group and 33 infants to gradual group (2 excluded for protocol deviation). In sudden and gradual groups, 14 and 22 infants, respectively, were weaned successfully in the first attempt (P=0.03). The infants were successfully weaned at 32.7±1.7 weeks versus 33.1±2.4 weeks (P=0.39) PMA and at a weight of 1651±290 g versus 1589±398 g (P=0.46) in the sudden and gradual groups, respectively. The total number of days on NCPAP was 27±19 days versus 32±24 days (P=0.38) and LOS was 63±25 days versus 63±22 days (P=0.99) in the sudden and gradual groups, respectively.
Conclusions: Gradual weaning method was more successful as compared to sudden weaning method in the initial trial off NCPAP. There was no difference in the PMA, weight at the time of successful wean, total days on NCPAP and LOS between the two groups.
Trial registration: ClinicalTrials.gov NCT02126501.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sudden vs Pressure Wean From Nasal Continuous Positive Airway Pressure in Infants Born Before 32 Weeks of Gestation: A Randomized Clinical Trial.JAMA Pediatr. 2018 Sep 1;172(9):824-831. doi: 10.1001/jamapediatrics.2018.2074. JAMA Pediatr. 2018. PMID: 30039171 Free PMC article. Clinical Trial.
-
Gradual versus sudden weaning from nasal CPAP in preterm infants: a pilot randomized controlled trial.Respir Care. 2013 Mar;58(3):511-6. doi: 10.4187/respcare.01999. Respir Care. 2013. PMID: 22906960 Clinical Trial.
-
Pressure versus Sudden Wean from Nasal Continuous Positive Airway Pressure in Preterm Infants: A Systematic Review and Meta-Analysis.Neonatology. 2020;117(5):537-544. doi: 10.1159/000507863. Epub 2020 Jun 24. Neonatology. 2020. PMID: 32580200
-
Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway pressure.BMC Pediatr. 2015 Oct 7;15:147. doi: 10.1186/s12887-015-0462-0. BMC Pediatr. 2015. PMID: 26446072 Free PMC article. Clinical Trial.
-
Weaning preterm infants from continuous positive airway pressure: evidence for best practice.World J Pediatr. 2015 Aug;11(3):212-8. doi: 10.1007/s12519-015-0022-6. Epub 2015 Apr 6. World J Pediatr. 2015. PMID: 25846068 Review.
Cited by
-
Eligible Infants Included in Neonatal Clinical Trials and Reasons for Noninclusion: A Systematic Review.JAMA Netw Open. 2024 Oct 1;7(10):e2441372. doi: 10.1001/jamanetworkopen.2024.41372. JAMA Netw Open. 2024. PMID: 39453652 Free PMC article.
-
Comparison of Two Methods for Weaning from Nasal Continuous Positive Airway Pressure via the Cyclic Use of High-Flow Nasal Cannula or Room Air in Preterm Infants.Children (Basel). 2024 Mar 15;11(3):351. doi: 10.3390/children11030351. Children (Basel). 2024. PMID: 38539386 Free PMC article.
-
Extended CPAP or low-flow nasal cannula for intermittent hypoxaemia in preterm infants: a 24-hour randomised clinical trial.Arch Dis Child Fetal Neonatal Ed. 2024 Aug 16;109(5):557-561. doi: 10.1136/archdischild-2023-326605. Arch Dis Child Fetal Neonatal Ed. 2024. PMID: 38365446 Free PMC article. Clinical Trial.
-
Use of noninvasive mechanical ventilation weaning protocol in neonatal intensive care units in Brazil: a descriptive study.Rev Paul Pediatr. 2023 May 15;41:e2021382. doi: 10.1590/1984-0462/2023/41/2021382. eCollection 2023. Rev Paul Pediatr. 2023. PMID: 37194837 Free PMC article.
-
Sudden vs Pressure Wean From Nasal Continuous Positive Airway Pressure in Infants Born Before 32 Weeks of Gestation: A Randomized Clinical Trial.JAMA Pediatr. 2018 Sep 1;172(9):824-831. doi: 10.1001/jamapediatrics.2018.2074. JAMA Pediatr. 2018. PMID: 30039171 Free PMC article. Clinical Trial.
References
-
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, et al. Nasal NCPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–708. - PubMed
-
- Aly H. Ventilation without tracheal intubation. Pediatrics. 2009;124:786–789. - PubMed
-
- Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069–e1076. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

