Building better biomarkers: brain models in translational neuroimaging
- PMID: 28230847
- PMCID: PMC5988350
- DOI: 10.1038/nn.4478
Building better biomarkers: brain models in translational neuroimaging
Abstract
Despite its great promise, neuroimaging has yet to substantially impact clinical practice and public health. However, a developing synergy between emerging analysis techniques and data-sharing initiatives has the potential to transform the role of neuroimaging in clinical applications. We review the state of translational neuroimaging and outline an approach to developing brain signatures that can be shared, tested in multiple contexts and applied in clinical settings. The approach rests on three pillars: (i) the use of multivariate pattern-recognition techniques to develop brain signatures for clinical outcomes and relevant mental processes; (ii) assessment and optimization of their diagnostic value; and (iii) a program of broad exploration followed by increasingly rigorous assessment of generalizability across samples, research contexts and populations. Increasingly sophisticated models based on these principles will help to overcome some of the obstacles on the road from basic neuroscience to better health and will ultimately serve both basic and applied goals.
Figures

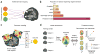
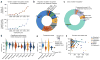


References
-
- Mather M, Cacioppo JT, Kanwisher N. Introduction to the special section: 20 years of fMRI-what has it done for understanding cognition? Perspect Psychol Sci. 2013;8:41–43. - PubMed
-
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. - PubMed
-
- Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. - PubMed
-
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. - PubMed
-
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

