Oncogenic role of rab escort protein 1 through EGFR and STAT3 pathway
- PMID: 28230863
- PMCID: PMC5386492
- DOI: 10.1038/cddis.2017.50
Oncogenic role of rab escort protein 1 through EGFR and STAT3 pathway
Abstract
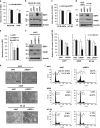
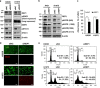
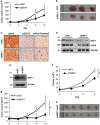
Rab escort protein-1 (REP1) is linked to choroideremia (CHM), an X-linked degenerative disorder caused by mutations of the gene encoding REP1 (CHM). REP1 mutant zebrafish showed excessive cell death throughout the body, including the eyes, indicating that REP1 is critical for cell survival, a hallmark of cancer. In the present study, we found that REP1 is overexpressed in human tumor tissues from cervical, lung, and colorectal cancer patients, whereas it is expressed at relatively low levels in the normal tissue counterparts. REP1 expression was also elevated in A549 lung cancer cells and HT-29 colon cancer cells compared with BEAS-2B normal lung and CCD-18Co normal colon epithelial cells, respectively. Interestingly, short interfering RNA (siRNA)-mediated REP1 knockdown-induced growth inhibition of cancer cell lines via downregulation of EGFR and inactivation of STAT3, but had a negligible effect on normal cell lines. Moreover, overexpression of REP1 in BEAS-2B cells enhanced cell growth and anchorage-independent colony formation with little increase in EGFR level and STAT3 activation. Furthermore, REP1 knockdown effectively reduced tumor growth in a mouse xenograft model via EGFR downregulation and STAT3 inactivation in vivo. These data suggest that REP1 plays an oncogenic role, driving tumorigenicity via EGFR and STAT3 signaling, and is a potential therapeutic target to control cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
REP1 inhibits FOXO3-mediated apoptosis to promote cancer cell survival.Cell Death Dis. 2017 Jan 5;8(1):e2536. doi: 10.1038/cddis.2016.462. Cell Death Dis. 2017. PMID: 28055019 Free PMC article.
-
Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo.J Mol Med (Berl). 2013 Jul;91(7):825-37. doi: 10.1007/s00109-013-1006-4. Epub 2013 Jun 12. J Mol Med (Berl). 2013. PMID: 23756766 Free PMC article.
-
LncRNA TSLNC8 synergizes with EGFR inhibitor osimertinib to inhibit lung cancer tumorigenesis by blocking the EGFR-STAT3 pathway.Cell Cycle. 2020 Nov;19(21):2776-2792. doi: 10.1080/15384101.2020.1820697. Epub 2020 Oct 16. Cell Cycle. 2020. PMID: 33064977 Free PMC article.
-
Rab escort protein 1 (REP1) in intracellular traffic: a functional and pathophysiological overview.Ophthalmic Genet. 2004 Jun;25(2):101-10. doi: 10.1080/13816810490514333. Ophthalmic Genet. 2004. PMID: 15370541 Review.
-
Choroideremia.Curr Opin Ophthalmol. 2017 Sep;28(5):410-415. doi: 10.1097/ICU.0000000000000392. Curr Opin Ophthalmol. 2017. PMID: 28520608 Review.
Cited by
-
Role of Rab GTPases in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2021 Nov 19;8:1389-1397. doi: 10.2147/JHC.S336251. eCollection 2021. J Hepatocell Carcinoma. 2021. PMID: 34824998 Free PMC article. Review.
-
Gene Expression and Transcriptome Profiling of Changes in a Cancer Cell Line Post-Exposure to Cadmium Telluride Quantum Dots: Possible Implications in Oncogenesis.Dose Response. 2021 Jun 11;19(2):15593258211019880. doi: 10.1177/15593258211019880. eCollection 2021 Apr-Jun. Dose Response. 2021. PMID: 34177396 Free PMC article.
-
Investigation of molecular mechanisms underlying JAK/STAT signaling pathway in HPV-induced cervical carcinogenesis using 'omics' approach.Med Oncol. 2022 Oct 12;39(12):255. doi: 10.1007/s12032-022-01854-1. Med Oncol. 2022. PMID: 36224441
-
CHML regulates migration and invasion in hepatocellular carcinoma via transcriptional and metabolic reprogramming.Front Oncol. 2025 Aug 6;15:1575809. doi: 10.3389/fonc.2025.1575809. eCollection 2025. Front Oncol. 2025. PMID: 40842592 Free PMC article.
-
REP1 Modulates Autophagy and Macropinocytosis to Enhance Cancer Cell Survival.Int J Mol Sci. 2017 Aug 28;18(9):1866. doi: 10.3390/ijms18091866. Int J Mol Sci. 2017. PMID: 28846638 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

