Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted
- PMID: 28231247
- PMCID: PMC5340401
- DOI: 10.1371/journal.pntd.0005389
Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted
Abstract
Background: In 2014-2015, we assessed favipiravir tolerance and efficacy in patients with Ebola virus (EBOV) disease (EVD) in Guinea (JIKI trial). Because the drug had never been used before for this indication and that high concentrations of the drugs were needed to achieve antiviral efficacy against EBOV, a pharmacokinetic model had been used to propose relevant dosing regimen. Here we report the favipiravir plasma concentrations that were achieved in participants in the JIKI trial and put them in perspective with the model-based targeted concentrations.
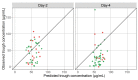
Methods and findings: Pre-dose drug concentrations were collected at Day-2 and Day-4 of treatment in 66 patients of the JIKI trial and compared to those predicted by the model taking into account patient's individual characteristics. At Day-2, the observed concentrations were slightly lower than the model predictions adjusted for patient's characteristics (median value of 46.1 versus 54.3 μg/mL for observed and predicted concentrations, respectively, p = 0.012). However, the concentrations dropped at Day-4, which was not anticipated by the model (median values of 25.9 and 64.4 μg/mL for observed and predicted concentrations, respectively, p<10-6). There was no significant relationship between favipiravir concentrations and EBOV viral kinetics or mortality.
Conclusions: Favipiravir plasma concentrations in the JIKI trial failed to achieve the target exposure defined before the trial. Furthermore, the drug concentration experienced an unanticipated drop between Day-2 and Day-4. The origin of this drop could be due to severe sepsis conditions and/or to intrinsic properties of favipiravir metabolism. Dose-ranging studies should be performed in healthy volunteers to assess the concentrations and the tolerance that could be achieved with high doses.
Trial registration: ClinicalTrials.gov NCT02329054.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: THTN, JG, VM, SB, HR, XdL and FM received a grant from St Luke International University (Tokyo, Japan) to perform research on favipiravir in non-human primates. All other authors declared no conflict of interest.
Figures



References
-
- World Health Organization. WHO | Ebola situation reports [Internet]. Available: http://apps.who.int/iris/bitstream/10665/208883/1/ebolasitrep_10Jun2016_...
-
- World Health Organization. Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola. In: Ebola treatments and interventions. 3 July 2015 [Internet]. [cited 7 Sep 2015]. Available: http://www.who.int/medicines/ebola-treatment/2015_0703TablesofEbolaDrugs...
-
- Madelain V, Nguyen THT, Olivo A, de Lamballerie X, Guedj J, Taburet A-M, et al. Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials. Clin Pharmacokinet. 2016;55: 907–923. 10.1007/s40262-015-0364-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

