Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice
- PMID: 28231312
- PMCID: PMC5322871
- DOI: 10.1371/journal.ppat.1006184
Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice
Abstract
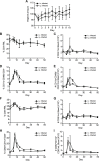
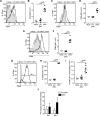
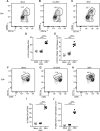
Zika virus (ZIKV) is an emerging arbovirus of the Flaviviridae family. Although ZIKV infection is typically mild and self-limiting in healthy adults, infection has been associated with neurological symptoms such as Guillain-Barré syndrome, and a causal link has been established between fetal microcephaly and ZIKV infection during pregnancy. These risks, and the magnitude of the ongoing ZIKV pandemic, have created an urgent need for the development of animal models to study the immune response to ZIKV infection. Previous animal models have primarily focused on pathogenesis in immunocompromised mice. In this study, we provide a model of ZIKV infection in wild-type immunocompetent C57BL/6 mice, and have provided an analysis of the immune response to infection. We evaluated the activation of several innate immune cell types, and studied the kinetics, phenotype, and functionality of T cell responses to ZIKV infection. Our results demonstrate that ZIKV infection is mild in wild-type immunocompetent C57BL/6 mice, resulting in minimal morbidity. Our data establish that at the peak of the adaptive response, antigen-experienced CD4+ T cells polarize to a Th1 phenotype, and antigen-experienced CD8+ T cells exhibit an activated effector phenotype, producing both effector cytokines and cytolytic molecules. Furthermore, we have identified a novel ZIKV CD8+ T cell epitope in the envelope protein that is recognized by the majority of responding cells. Our model provides an important reference point that will help dissect the impact of polymorphisms in the circulating ZIKV strains on the immune response and ZIKV pathogenesis. In addition, the identification of a ZIKV epitope will allow for the design of tetramers to study epitope-specific T cell responses, and will have important implications for the design and development of ZIKV vaccine strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
CD8+ T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection.J Virol. 2017 Oct 27;91(22):e00900-17. doi: 10.1128/JVI.00900-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28835502 Free PMC article.
-
In silico construction of a multiepitope Zika virus vaccine using immunoinformatics tools.Sci Rep. 2022 Jan 7;12(1):53. doi: 10.1038/s41598-021-03990-6. Sci Rep. 2022. PMID: 34997041 Free PMC article.
-
Identification of Protective CD8 T Cell Responses in a Mouse Model of Zika Virus Infection.Front Immunol. 2019 Jul 17;10:1678. doi: 10.3389/fimmu.2019.01678. eCollection 2019. Front Immunol. 2019. PMID: 31379867 Free PMC article.
-
Using immunocompromised mice to identify mechanisms of Zika virus transmission and pathogenesis.Immunology. 2018 Apr;153(4):443-454. doi: 10.1111/imm.12883. Epub 2018 Jan 19. Immunology. 2018. PMID: 29266213 Free PMC article. Review.
-
Protective to a T: The Role of T Cells during Zika Virus Infection.Cells. 2019 Aug 3;8(8):820. doi: 10.3390/cells8080820. Cells. 2019. PMID: 31382545 Free PMC article. Review.
Cited by
-
A viral-specific CD4+ T cell response protects female mice from Coxsackievirus B3 infection.Front Immunol. 2024 Jan 11;14:1327384. doi: 10.3389/fimmu.2023.1327384. eCollection 2023. Front Immunol. 2024. PMID: 38274806 Free PMC article.
-
Differential Longevity of Memory CD4 and CD8 T Cells in a Cohort of the Mothers With a History of ZIKV Infection and Their Children.Front Immunol. 2021 Feb 12;12:610456. doi: 10.3389/fimmu.2021.610456. eCollection 2021. Front Immunol. 2021. PMID: 33679748 Free PMC article.
-
Viral Infections Exacerbate FUS-ALS Phenotypes in iPSC-Derived Spinal Neurons in a Virus Species-Specific Manner.Front Cell Neurosci. 2019 Oct 22;13:480. doi: 10.3389/fncel.2019.00480. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31695598 Free PMC article.
-
Zika virus baculovirus-expressed envelope protein elicited humoral and cellular immunity in immunocompetent mice.Sci Rep. 2022 Jan 13;12(1):660. doi: 10.1038/s41598-021-04713-7. Sci Rep. 2022. PMID: 35027643 Free PMC article.
-
A New In Vivo Model to Study Protective Immunity to Zika Virus Infection in Mice With Intact Type I Interferon Signaling.Front Immunol. 2018 Mar 22;9:593. doi: 10.3389/fimmu.2018.00593. eCollection 2018. Front Immunol. 2018. PMID: 29623081 Free PMC article.
References
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. - PubMed
-
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–34. - PubMed
-
- Simpson DI. Zika Virus Infection in Man. Trans R Soc Trop Med Hyg. 1964;58:335–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

