Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons
- PMID: 28231468
- PMCID: PMC5340420
- DOI: 10.1016/j.neuron.2017.01.019
Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons
Abstract
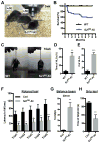
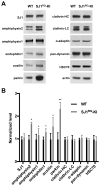
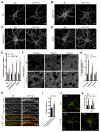
Synaptojanin 1 (SJ1) is a major presynaptic phosphatase that couples synaptic vesicle endocytosis to the dephosphorylation of PI(4,5)P2, a reaction needed for the shedding of endocytic factors from their membranes. While the role of SJ1's 5-phosphatase module in this process is well recognized, the contribution of its Sac phosphatase domain, whose preferred substrate is PI4P, remains unclear. Recently a homozygous mutation in its Sac domain was identified in early-onset parkinsonism patients. We show that mice carrying this mutation developed neurological manifestations similar to those of human patients. Synapses of these mice displayed endocytic defects and a striking accumulation of clathrin-coated intermediates, strongly implicating Sac domain's activity in endocytic protein dynamics. Mutant brains had elevated auxilin (PARK19) and parkin (PARK2) levels. Moreover, dystrophic axonal terminal changes were selectively observed in dopaminergic axons in the dorsal striatum. These results strengthen evidence for a link between synaptic endocytic dysfunction and Parkinson's disease.
Keywords: LRRK2; PARK19; PARK2; PARK20; PI(4,5)P2; Parkin; auxilin; neurodegeneration; nigrostriatal pathway; synaptic vesicle endocytosis; synaptojanin 1.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Parkinson disease and clathrin coat dynamics at synapses, why not?Mov Disord. 2017 Aug;32(8):1163. doi: 10.1002/mds.27097. Epub 2017 Jul 6. Mov Disord. 2017. PMID: 28681957 Free PMC article. No abstract available.
Similar articles
-
Parkinsonism Sac domain mutation in Synaptojanin-1 affects ciliary properties in iPSC-derived dopaminergic neurons.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2318943121. doi: 10.1073/pnas.2318943121. Epub 2024 Apr 18. Proc Natl Acad Sci U S A. 2024. PMID: 38635628 Free PMC article.
-
Absence of Sac2/INPP5F enhances the phenotype of a Parkinson's disease mutation of synaptojanin 1.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12428-12434. doi: 10.1073/pnas.2004335117. Epub 2020 May 18. Proc Natl Acad Sci U S A. 2020. PMID: 32424101 Free PMC article.
-
Dysfunction of synaptic endocytic trafficking in Parkinson's disease.Neural Regen Res. 2024 Dec 1;19(12):2649-2660. doi: 10.4103/NRR.NRR-D-23-01624. Epub 2024 Mar 1. Neural Regen Res. 2024. PMID: 38595283 Free PMC article.
-
Endophilin and synaptojanin hook up to promote synaptic vesicle endocytosis.Neuron. 2003 Nov 13;40(4):665-7. doi: 10.1016/s0896-6273(03)00726-8. Neuron. 2003. PMID: 14622570 Review.
-
Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse.Biochem Soc Trans. 2006 Feb;34(Pt 1):68-72. doi: 10.1042/BST0340068. Biochem Soc Trans. 2006. PMID: 16417485 Free PMC article. Review.
Cited by
-
Mini-review: Synaptojanin 1 and its implications in membrane trafficking.Neurosci Lett. 2021 Nov 20;765:136288. doi: 10.1016/j.neulet.2021.136288. Epub 2021 Oct 9. Neurosci Lett. 2021. PMID: 34637856 Free PMC article. Review.
-
Gait Analysis of Age-dependent Motor Impairments in Mice with Neurodegeneration.J Vis Exp. 2018 Jun 18;(136):57752. doi: 10.3791/57752. J Vis Exp. 2018. PMID: 29985360 Free PMC article.
-
An Emerging Role for Phosphoinositides in the Pathophysiology of Parkinson's Disease.J Parkinsons Dis. 2021;11(4):1725-1750. doi: 10.3233/JPD-212684. J Parkinsons Dis. 2021. PMID: 34151859 Free PMC article. Review.
-
Periplaneta americana L. extract exerts neuroprotective effects by inhibiting endoplasmic reticulum stress via AKT-dependent pathway in experimental models of Parkinson's disease.Chin Med. 2024 Nov 13;19(1):157. doi: 10.1186/s13020-024-01029-2. Chin Med. 2024. PMID: 39538357 Free PMC article.
-
A Critical LRRK at the Synapse? The Neurobiological Function and Pathophysiological Dysfunction of LRRK2.Front Mol Neurosci. 2020 Aug 27;13:153. doi: 10.3389/fnmol.2020.00153. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32973447 Free PMC article. Review.
References
-
- Arranz AM, Delbroek L, Van Kolen K, Guimaraes MR, Mandemakers W, Daneels G, Matta S, Calafate S, Shaban H, Baatsen P, et al. LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. Journal of cell science. 2015;128:541–552. - PubMed
-
- Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2626–2631. - PMC - PubMed
-
- Boassa D, Berlanga ML, Yang MA, Terada M, Hu J, Bushong EA, Hwang M, Masliah E, George JM, Ellisman MH. Mapping the subcellular distribution of alpha-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson’s disease pathogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:2605–2615. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

