The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway
- PMID: 28231612
- PMCID: PMC5404961
- DOI: 10.1111/micc.12364
The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway
Abstract
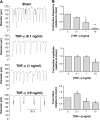
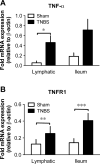
Objective: Mesenteric lymphatic vessel pumping, important to propel lymph and immune cells from the intestinal interstitium to the mesenteric lymph nodes, is compromised during intestinal inflammation. The objective of this study was to test the hypothesis that the pro-inflammatory cytokine TNF-α, is a significant contributor to the inflammation-induced lymphatic contractile dysfunction, and to determine its mode of action.
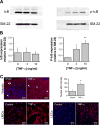
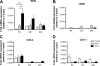
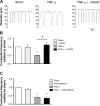
Methods: Contractile parameters were obtained from isolated rat mesenteric lymphatic vessels mounted on a pressure myograph after 24-hours incubation with or without TNF-α. Various inhibitors were administered, and quantitative real-time PCR, Western blotting, and immunofluorescence confocal imaging were applied to characterize the mechanisms involved in TNF-α actions.
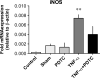
Results: Vessel contraction frequency was significantly decreased after TNF-α treatment and could be restored by selective inhibition of NF-кB, iNOS, guanylate cyclase, and ATP-sensitive K+ channels. We further demonstrated that NF-кB inhibition also suppressed the significant increase in iNOS mRNA observed in TNF-α-treated lymphatic vessels and that TNF-α treatment favored the nuclear translocation of the p65 NF-κB subunit.
Conclusions: These findings suggest that TNF-α decreases mesenteric lymphatic contractility by activating the NF-κB-iNOS signaling pathway. This mechanism could contribute to the alteration of lymphatic pumping reported in intestinal inflammation.
Keywords: cytokine; inflammation; lymphatic vessel contraction; nitric oxide synthase; nuclear factor-kappa B.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures

Similar articles
-
Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis.Am J Physiol Gastrointest Liver Physiol. 2013 Mar 15;304(6):G623-34. doi: 10.1152/ajpgi.00392.2012. Epub 2012 Dec 28. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23275612
-
Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice.Arthritis Res Ther. 2016 Mar 12;18:62. doi: 10.1186/s13075-016-0963-8. Arthritis Res Ther. 2016. PMID: 26970913 Free PMC article.
-
Glycyrrhetinic acid suppressed NF-κB activation in TNF-α-induced hepatocytes.J Agric Food Chem. 2014 Jan 22;62(3):618-25. doi: 10.1021/jf405352g. Epub 2014 Jan 10. J Agric Food Chem. 2014. PMID: 24386942
-
Aged Lymphatic Vessels and Mast Cells in Perilymphatic Tissues.Int J Mol Sci. 2017 May 3;18(5):965. doi: 10.3390/ijms18050965. Int J Mol Sci. 2017. PMID: 28467354 Free PMC article. Review.
-
Lymphatic vessels in health and disease.Wiley Interdiscip Rev Syst Biol Med. 2013 Jan-Feb;5(1):111-24. doi: 10.1002/wsbm.1201. Epub 2012 Dec 3. Wiley Interdiscip Rev Syst Biol Med. 2013. PMID: 23209022 Free PMC article. Review.
Cited by
-
Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology.Front Immunol. 2020 Jun 17;11:1234. doi: 10.3389/fimmu.2020.01234. eCollection 2020. Front Immunol. 2020. PMID: 32625213 Free PMC article. Review.
-
Lymphatic dysfunction correlates with inflammation in a mouse model of amyotrophic lateral sclerosis.Dis Model Mech. 2025 Jul 1;18(7):dmm052148. doi: 10.1242/dmm.052148. Epub 2025 Jul 16. Dis Model Mech. 2025. PMID: 40600271 Free PMC article.
-
Obstructive Lymphangitis Precedes Colitis in Murine Norovirus-Infected Stat1-Deficient Mice.Am J Pathol. 2018 Jul;188(7):1536-1554. doi: 10.1016/j.ajpath.2018.03.019. Epub 2018 May 18. Am J Pathol. 2018. PMID: 29753791 Free PMC article.
-
Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis.Nat Rev Rheumatol. 2018 Feb;14(2):94-106. doi: 10.1038/nrrheum.2017.205. Epub 2018 Jan 11. Nat Rev Rheumatol. 2018. PMID: 29323343 Free PMC article. Review.
-
Evaluation of the degree and distribution of lymphangiectasia in full-thickness canine small intestinal specimens diagnosed with lymphoplasmacytic enteritis and granulomatous lymphangitis.J Vet Med Sci. 2022 Apr 15;84(4):566-573. doi: 10.1292/jvms.21-0257. Epub 2022 Mar 14. J Vet Med Sci. 2022. PMID: 35283405 Free PMC article.
References
-
- Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. - PubMed
-
- Almodovar R, Zarco P, Quiros FJ, Mazzucchelli R. Infliximab treatment efficacy in lymphoedema associated with ankylosing spondylitis. Rheumatology (Oxford) 2004;43:1456. - PubMed
-
- Arias-Negrete S, Keller K, Chadee K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem Biophys Res Commun. 1995;208:582–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

