Research waste in diagnostic trials: a methods review evaluating the reporting of test-treatment interventions
- PMID: 28231757
- PMCID: PMC5324286
- DOI: 10.1186/s12874-016-0286-0
Research waste in diagnostic trials: a methods review evaluating the reporting of test-treatment interventions
Abstract
Background: The most rigorous method for evaluating the effectiveness of diagnostic tests is through randomised trials that compare test-treatment interventions: complex interventions comprising episodes of testing, decision-making and treatment. The multi-staged nature of these interventions, combined with the need to relay diagnostic decision-making and treatment planning, has led researchers to hypothesise that test-treatment strategies may be very challenging to document. However, no reviews have yet examined the reporting quality of interventions used in test-treatment RCTs. In this study we evaluate the completeness of intervention descriptions in a systematically identified cohort of test-treatment RCTs.
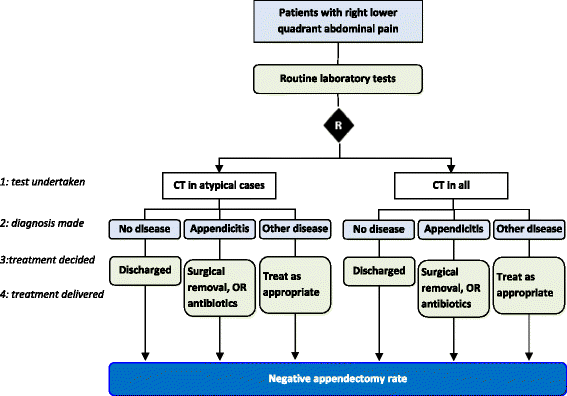
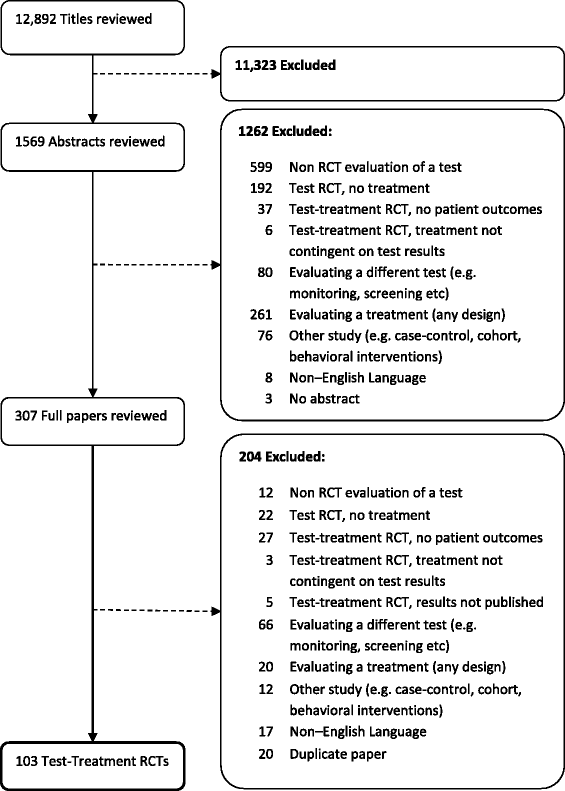
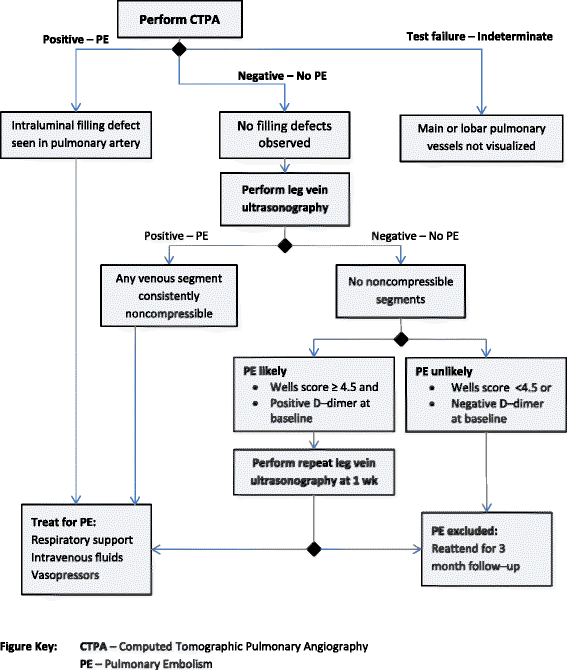
Methods: We ascertained all test-treatment RCTs published 2004-2007, indexed in CENTRAL. Included trials randomized patients to diagnostic tests and measured patient outcomes after treatment. Two raters examined the completeness of test-treatment intervention descriptions in four components: 1) the test, 2) diagnostic decision-making, 3) management decision-making, 4) treatments.
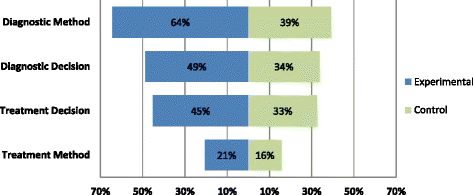
Results: One hundred and three trials compared 105 control with 119 experimental interventions, most commonly in cardiovascular medicine (35, 34%), obstetrics and gynecology (17%), gastroenterology (14%) or orthopedics (10%). A broad range of tests were evaluated, including imaging (50, 42%), biochemical assays (21%) and clinical assessment (12%). Only five (5%) trials detailed all four components of experimental and control interventions, none of which also provided a complete care pathway diagram. Experimental arms were missing descriptions of tests, diagnostic-decision making, management planning and treatments (36%, 51%, 55% and 79% of trials respectively); control arms were missing the same details in 61%, 66%, 67% and 84% of trials.
Conclusion: Reporting of test-treatment interventions is very poor, inadequate for understanding the results of these trials, and for comparing or translating results into clinical practice. Reporting needs to improve, with greater emphasis on describing the decision-making components of care pathways in both pragmatic and explanatory trials. Please see the companion paper to this article: http://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-016-0287-z .
Keywords: Diagnostic accuracy; Intervention reporting; Patient outcomes; RCT; Reporting quality; Test Evaluation; Test-treatment.
Figures
Similar articles
-
Test-treatment RCTs are susceptible to bias: a review of the methodological quality of randomized trials that evaluate diagnostic tests.BMC Med Res Methodol. 2017 Feb 24;17(1):35. doi: 10.1186/s12874-016-0287-z. BMC Med Res Methodol. 2017. PMID: 28236806 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Assessment of the completeness of intervention reporting of randomized clinical trials for alcohol use disorders: Effect of the TIDieR checklist and guide.Drug Alcohol Depend. 2020 Mar 1;208:107824. doi: 10.1016/j.drugalcdep.2019.107824. Epub 2020 Jan 28. Drug Alcohol Depend. 2020. PMID: 32014645 Review.
-
How should we evaluate the risk of bias of physical therapy trials?: a psychometric and meta-epidemiological approach towards developing guidelines for the design, conduct, and reporting of RCTs in Physical Therapy (PT) area: a study protocol.Syst Rev. 2013 Sep 26;2:88. doi: 10.1186/2046-4053-2-88. Syst Rev. 2013. PMID: 24070072 Free PMC article.
Cited by
-
Accuracy of pre-hospital triage tools for major trauma: a systematic review with meta-analysis and net clinical benefit.World J Emerg Surg. 2021 Jun 10;16(1):31. doi: 10.1186/s13017-021-00372-1. World J Emerg Surg. 2021. PMID: 34112209 Free PMC article.
-
Randomized test-treatment studies with an outlook on adaptive designs.BMC Med Res Methodol. 2021 Jun 1;21(1):110. doi: 10.1186/s12874-021-01293-y. BMC Med Res Methodol. 2021. PMID: 34074263 Free PMC article.
-
Test-treatment RCTs are susceptible to bias: a review of the methodological quality of randomized trials that evaluate diagnostic tests.BMC Med Res Methodol. 2017 Feb 24;17(1):35. doi: 10.1186/s12874-016-0287-z. BMC Med Res Methodol. 2017. PMID: 28236806 Free PMC article. Review.
-
A novel approach to sharing all available information from funded health research: the NIHR Journals Library.Health Res Policy Syst. 2018 Jul 31;16(1):70. doi: 10.1186/s12961-018-0339-4. Health Res Policy Syst. 2018. PMID: 30064444 Free PMC article.
-
A review identified challenges distinguishing primary reports of randomized trials for meta-research: A proposal for improved reporting.J Clin Epidemiol. 2022 May;145:121-125. doi: 10.1016/j.jclinepi.2022.01.013. Epub 2022 Jan 23. J Clin Epidemiol. 2022. PMID: 35081448 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

