Malaria parasite clearance
- PMID: 28231817
- PMCID: PMC5324257
- DOI: 10.1186/s12936-017-1731-1
Malaria parasite clearance
Erratum in
-
Erratum to: Malaria parasite clearance.Malar J. 2017 May 10;16(1):194. doi: 10.1186/s12936-017-1785-0. Malar J. 2017. PMID: 28490366 Free PMC article. No abstract available.
Abstract
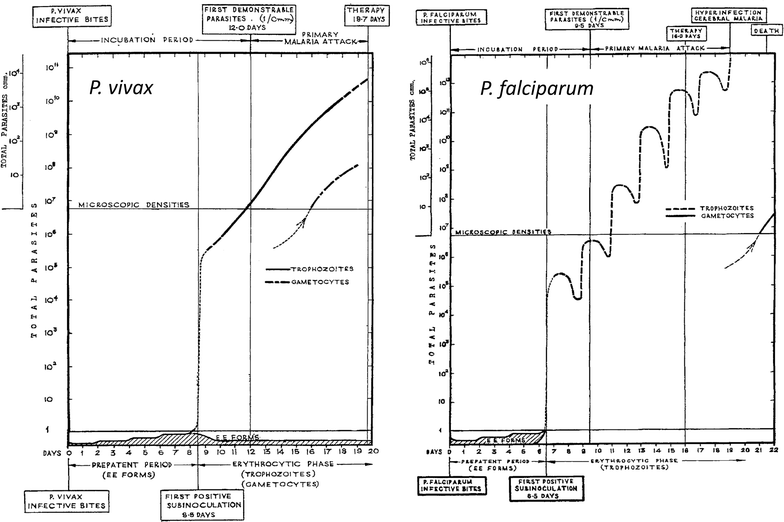
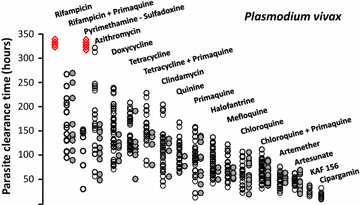
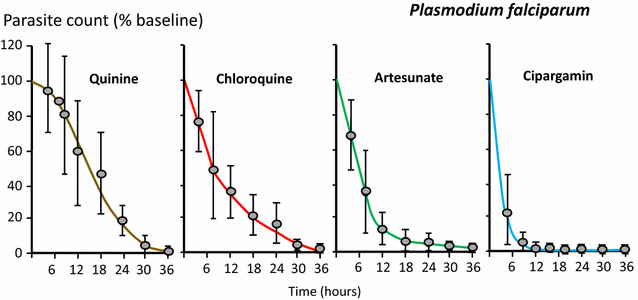
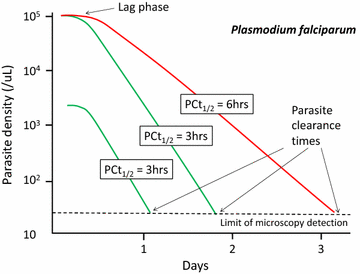
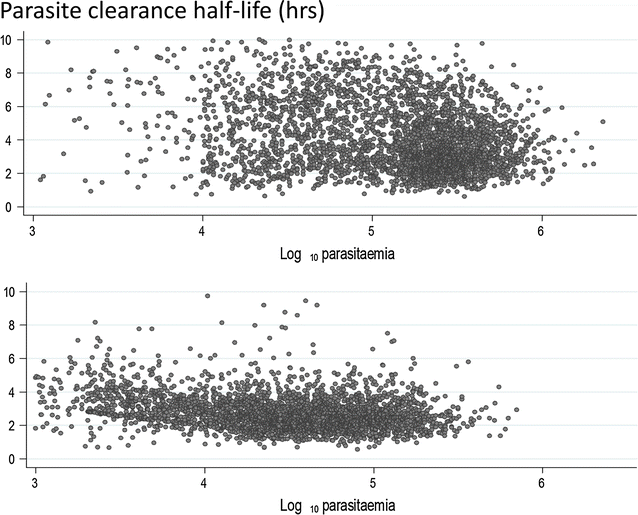
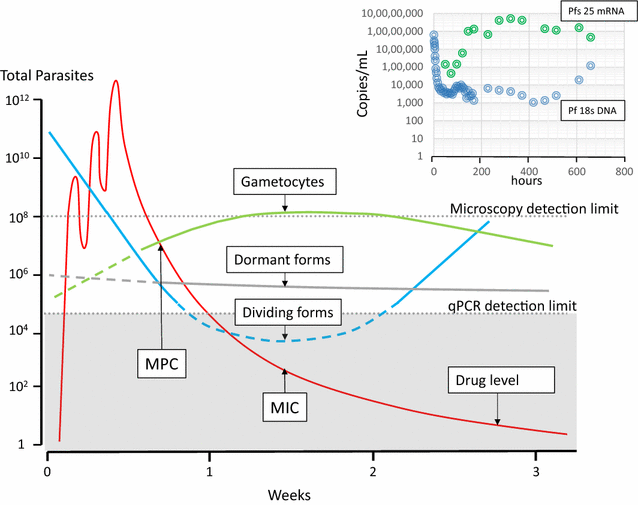
Following anti-malarial drug treatment asexual malaria parasite killing and clearance appear to be first order processes. Damaged malaria parasites in circulating erythrocytes are removed from the circulation mainly by the spleen. Splenic clearance functions increase markedly in acute malaria. Either the entire infected erythrocytes are removed because of their reduced deformability or increased antibody binding or, for the artemisinins which act on young ring stage parasites, splenic pitting of drug-damaged parasites is an important mechanism of clearance. The once-infected erythrocytes returned to the circulation have shortened survival. This contributes to post-artesunate haemolysis that may follow recovery in non-immune hyperparasitaemic patients. As the parasites mature Plasmodium vivax-infected erythrocytes become more deformable, whereas Plasmodium falciparum-infected erythrocytes become less deformable, but they escape splenic filtration by sequestering in venules and capillaries. Sequestered parasites are killed in situ by anti-malarial drugs and then disintegrate to be cleared by phagocytic leukocytes. After treatment with artemisinin derivatives some asexual parasites become temporarily dormant within their infected erythrocytes, and these may regrow after anti-malarial drug concentrations decline. Artemisinin resistance in P. falciparum reflects reduced ring stage susceptibility and manifests as slow parasite clearance. This is best assessed from the slope of the log-linear phase of parasitaemia reduction and is commonly measured as a parasite clearance half-life. Pharmacokinetic-pharmacodynamic modelling of anti-malarial drug effects on parasite clearance has proved useful in predicting therapeutic responses and in dose-optimization.
Figures
References
-
- World Health Organization Severe malaria. Trop Med Int Health. 2014;19(Supplement 1):1–131.
-
- Marchiafava E, Bignami A. On summer-autumnal fever. London: New Sydenham Society; 1894.
-
- WHO . Guidelines for the treatment of malaria. 3. Geneva: World Health Organization; 2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

