Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy
- PMID: 28231823
- PMCID: PMC5324197
- DOI: 10.1186/s12883-017-0790-9
Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy
Abstract
Background: Reports on the clinical meaningfulness of outcome measures in spinal muscular atrophy (SMA) are rare. In this two-part study, our aim was to explore patients' and caregivers' views on the clinical relevance of the Hammersmith Functional Motor Scale Expanded- (HFMSE).
Methods: First, we used focus groups including SMA patients and caregivers to explore their views on the clinical relevance of the individual activities included in the HFMSE. Then we asked caregivers to comment on the clinical relevance of possible changes of HFMSE scores over time. As functional data of individual patients were available, some of the questions were tailored according to their functional level on the HFMSE.
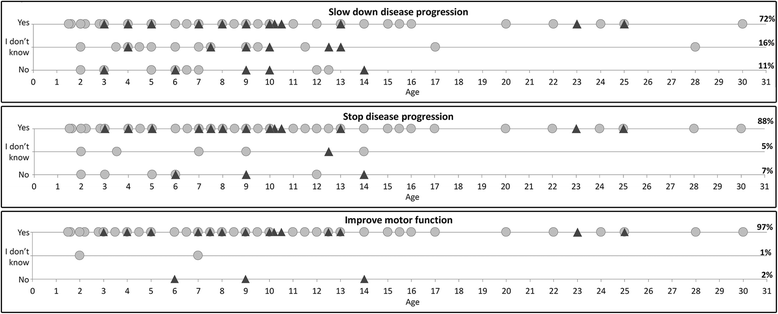
Results: Part 1: Sixty-three individuals participated in the focus groups. This included 30 caregivers, 25 patients and 8 professionals who facilitated the discussion. The caregivers provided a comparison to activities of daily living for each of the HFMSE items. Part 2: One hundred and forty-nine caregivers agreed to complete the questionnaire: in response to a general question, 72% of the caregivers would consider taking part in a clinical trial if the treatment was expected to slow down deterioration, 88% if it would stop deterioration and 97% if the treatment was expected to produce an improvement. Caregivers were informed of the first three items that their child could not achieve on the HFMSE. In response 75% indicated a willingness to take part in a clinical trial if they could achieve at least one of these abilities, 89% if they could achieve two, and 100% if they could achieve more than 2.
Conclusions: Our findings support the use of the HFMSE as a key outcome measure in SMA clinical trials because the individual items and the detected changes have clear content validity and clinical meaningfulness for patients and their caregivers.
Keywords: Carers; Clinical trials; Quality of life; Spinal muscular atrophy.
Figures



Similar articles
-
Nusinersen in Adults with 5q Spinal Muscular Atrophy: a Systematic Review and Meta-analysis.Neurotherapeutics. 2022 Mar;19(2):464-475. doi: 10.1007/s13311-022-01200-3. Epub 2022 Feb 17. Neurotherapeutics. 2022. PMID: 35178673 Free PMC article.
-
A qualitative study of perceptions of meaningful change in spinal muscular atrophy.BMC Neurol. 2017 Apr 4;17(1):68. doi: 10.1186/s12883-017-0853-y. BMC Neurol. 2017. PMID: 28376816 Free PMC article.
-
Nusinersen for adults with spinal muscular atrophy.Neurol Sci. 2023 Jul;44(7):2393-2400. doi: 10.1007/s10072-023-06698-9. Epub 2023 Mar 1. Neurol Sci. 2023. PMID: 36854931
-
Understanding the relationship between the 32-item motor function measure and daily activities from an individual with spinal muscular atrophy and their caregivers' perspective: a two-part study.BMC Neurol. 2021 Mar 31;21(1):143. doi: 10.1186/s12883-021-02166-z. BMC Neurol. 2021. PMID: 33789607 Free PMC article.
-
Drug treatment for spinal muscular atrophy types II and III.Cochrane Database Syst Rev. 2020 Jan 6;1(1):CD006282. doi: 10.1002/14651858.CD006282.pub5. Cochrane Database Syst Rev. 2020. PMID: 32006461 Free PMC article.
Cited by
-
Effects of nusinersen on motor function in children with spinal muscular atrophy: a retrospective study.Front Neurol. 2024 Jul 15;15:1391613. doi: 10.3389/fneur.2024.1391613. eCollection 2024. Front Neurol. 2024. PMID: 39076847 Free PMC article.
-
Assessment of motor function and nutritional status in children with spinal muscular atrophy treated with nusinersen after loading period in Western China: a retrospective study.BMC Neurol. 2023 Jan 23;23(1):35. doi: 10.1186/s12883-023-03063-3. BMC Neurol. 2023. PMID: 36690929 Free PMC article.
-
Nusinersen in Adults with 5q Spinal Muscular Atrophy: a Systematic Review and Meta-analysis.Neurotherapeutics. 2022 Mar;19(2):464-475. doi: 10.1007/s13311-022-01200-3. Epub 2022 Feb 17. Neurotherapeutics. 2022. PMID: 35178673 Free PMC article.
-
Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies.Neurology. 2019 May 21;92(21):e2492-e2506. doi: 10.1212/WNL.0000000000007527. Epub 2019 Apr 24. Neurology. 2019. PMID: 31019106 Free PMC article. Clinical Trial.
-
Muscle quantitative MRI in adult SMA patients on nusinersen treatment: a longitudinal study.Acta Myol. 2022 Jun 30;41(2):76-83. doi: 10.36185/2532-1900-074. eCollection 2022 Jun. Acta Myol. 2022. PMID: 35832502 Free PMC article.
References
-
- O’Hagen JM, Glanzman AM, McDermott MP, Ryan PA, Flickinger J, Quigley J, Riley S, Sanborn E, Irvine C, Martens WB, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. 2007;17(9-10):693–697. doi: 10.1016/j.nmd.2007.05.009. - DOI - PubMed
-
- Mercuri E, Messina S, Battini R, Berardinelli A, Boffi P, Bono R, Bruno C, Carboni N, Cini C, Colitto F, et al. Reliability of the Hammersmith functional motor scale for spinal muscular atrophy in a multicentric study. Neuromuscul Disord. 2006;16(2):93–98. doi: 10.1016/j.nmd.2005.11.010. - DOI - PubMed
-
- Mercuri E, Mayhew A, Muntoni F, Messina S, Straub V, Van Ommen GJ, Voit T, Bertini E, Bushby K. Towards harmonisation of outcome measures for DMD and SMA within TREAT-NMD; report of three expert workshops: TREAT-NMD/ENMC workshop on outcome measures, 12th--13th May 2007, Naarden, The Netherlands; TREAT-NMD workshop on outcome measures in experimental trials for DMD, 30th June--1st July 2007, Naarden, The Netherlands; conjoint Institute of Myology TREAT-NMD meeting on physical activity monitoring in neuromuscular disorders, 11th July 2007, Paris, France. Neuromuscul Disord. 2008;18(11):894–903. doi: 10.1016/j.nmd.2008.07.003. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

