Assessment and rehabilitation of central sensory impairments for balance in mTBI using auditory biofeedback: a randomized clinical trial
- PMID: 28231824
- PMCID: PMC5324311
- DOI: 10.1186/s12883-017-0812-7
Assessment and rehabilitation of central sensory impairments for balance in mTBI using auditory biofeedback: a randomized clinical trial
Abstract
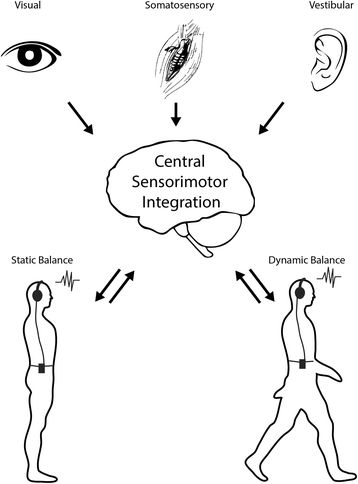
Background: Complaints of imbalance are common non-resolving signs in individuals with post-concussive syndrome. Yet, there is no consensus rehabilitation for non-resolving balance complaints following mild traumatic brain injury (mTBI). The heterogeneity of balance deficits and varied rates of recovery suggest varied etiologies and a need for interventions that address the underlying causes of poor balance function. Our central hypothesis is that most chronic balance deficits after mTBI result from impairments in central sensorimotor integration that may be helped by rehabilitation. Two studies are described to 1) characterize balance deficits in people with mTBI who have chronic, non-resolving balance deficits compared to healthy control subjects, and 2) determine the efficacy of an augmented vestibular rehabilitation program using auditory biofeedback to improve central sensorimotor integration, static and dynamic balance, and functional activity in patients with chronic mTBI.
Methods: Two studies are described. Study 1 is a cross-sectional study to take place jointly at Oregon Health and Science University and the VA Portland Health Care System. The study participants will be individuals with non-resolving complaints of balance following mTBI and age- and gender-matched controls who meet all inclusion criteria. The primary outcome will be measures of central sensorimotor integration derived from a novel central sensorimotor integration test. Study 2 is a randomized controlled intervention to take place at Oregon Health & Science University. In this study, participants from Study 1 with mTBI and abnormal central sensorimotor integration will be randomized into two rehabilitation interventions. The interventions will be 6 weeks of vestibular rehabilitation 1) with or 2) without the use of an auditory biofeedback device. The primary outcome measure is the daily activity of the participants measured using an inertial sensor.
Discussion: The results of these two studies will improve our understanding of the nature of balance deficits in people with mTBI by providing quantitative metrics of central sensorimotor integration, balance, and vestibular and ocular motor function. Study 2 will examine the potential for augmented rehabilitation interventions to improve central sensorimotor integration.
Trial registration: This trial is registered at clinicaltrials.gov ( NCT02748109 ).
Keywords: Balance; Biofeedback; Concussion; Gait; Sensorimotor integration; mTBI.
Figures


Similar articles
-
The effects of augmenting traditional rehabilitation with audio biofeedback in people with persistent imbalance following mild traumatic brain injury.Front Neurol. 2022 Oct 4;13:926691. doi: 10.3389/fneur.2022.926691. eCollection 2022. Front Neurol. 2022. PMID: 36267889 Free PMC article.
-
The Sensor Technology and Rehabilitative Timing (START) Protocol: A Randomized Controlled Trial for the Rehabilitation of Mild Traumatic Brain Injury.Phys Ther. 2020 Apr 17;100(4):687-697. doi: 10.1093/ptj/pzaa007. Phys Ther. 2020. PMID: 31951263 Free PMC article.
-
Assessing subacute mild traumatic brain injury with a portable virtual reality balance device.Disabil Rehabil. 2017 Jul;39(15):1564-1572. doi: 10.1080/09638288.2016.1226432. Epub 2016 Oct 10. Disabil Rehabil. 2017. PMID: 27718642
-
Vestibulo-ocular dysfunction in mTBI: Utility of the VOMS for evaluation and management - A review.NeuroRehabilitation. 2022;50(3):279-296. doi: 10.3233/NRE-228012. NeuroRehabilitation. 2022. PMID: 35311725 Review.
-
Vestibular Rehabilitation Effectiveness for Adults With Mild Traumatic Brain Injury/Concussion: A Mini-Systematic Review.Am J Audiol. 2022 Mar 3;31(1):228-242. doi: 10.1044/2021_AJA-21-00165. Epub 2022 Jan 25. Am J Audiol. 2022. PMID: 35077655
Cited by
-
The effects of augmenting traditional rehabilitation with audio biofeedback in people with persistent imbalance following mild traumatic brain injury.Front Neurol. 2022 Oct 4;13:926691. doi: 10.3389/fneur.2022.926691. eCollection 2022. Front Neurol. 2022. PMID: 36267889 Free PMC article.
-
Effectiveness of non-pharmacological treatments for vestibular and oculomotor dysfunction in patients with persistent post-concussive symptoms: protocol for a systematic review and meta-analysis.BMJ Open. 2023 Jan 6;13(1):e066634. doi: 10.1136/bmjopen-2022-066634. BMJ Open. 2023. PMID: 36609322 Free PMC article.
-
Evaluation of cerebellum volume and trunk oscillation velocity in cases with adolescent idiopathic scoliosis: a preliminary report.Eur Spine J. 2023 Nov;32(11):4012-4019. doi: 10.1007/s00586-023-07948-2. Epub 2023 Sep 19. Eur Spine J. 2023. PMID: 37725163
-
Abnormal Turning and Its Association with Self-Reported Symptoms in Chronic Mild Traumatic Brain Injury.J Neurotrauma. 2018 May 15;35(10):1167-1177. doi: 10.1089/neu.2017.5231. Epub 2018 Mar 23. J Neurotrauma. 2018. PMID: 29078732 Free PMC article.
-
Gait and balance disturbances are common in young urbanites and associated with cognitive impairment. Air pollution and the historical development of Alzheimer's disease in the young.Environ Res. 2020 Dec;191:110087. doi: 10.1016/j.envres.2020.110087. Epub 2020 Sep 2. Environ Res. 2020. PMID: 32890478 Free PMC article.
References
-
- Boake C, McCauley SR, Levin HS, Pedroza C, Contant CF, Song JX, Brown SA, Goodman H, Brundage SI, Diaz-Marchan PJ. Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(3):350–356. doi: 10.1176/jnp.17.3.350. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

