What is the optimal sequence of decompression for multilevel noncontinuous spinal cord compression injuries in rabbits?
- PMID: 28231826
- PMCID: PMC5324218
- DOI: 10.1186/s12883-017-0824-3
What is the optimal sequence of decompression for multilevel noncontinuous spinal cord compression injuries in rabbits?
Abstract
Background: In recent years, multilevel spinal cord injuries (SCIs) have gained a substantial amount of attention from clinicians and researchers. Multilevel noncontinuous SCI patients cannot undergo the multiple steps of a one-stage operation because of a poor general condition or a lack of proper surgical approaches. The surgeon subsequently faces the decision of whether to initially relieve the rostral or caudal compression. In this study, we established a spinal cord compression model involving two noncontinuous segments in rabbits to evaluate the effects of differences in decompression order on the functional recovery of the spinal cord.
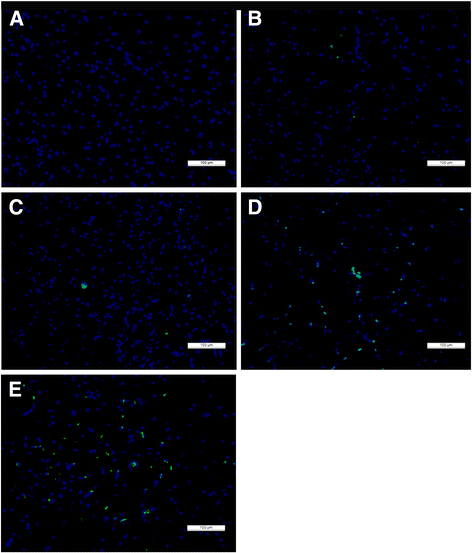
Methods: A Fogarty catheter was inserted into the epidural space through a hole in T6-7 and advanced 3 cm rostrally or caudally. Following successful model establishment, which was demonstrated by an evaluation of evoked potentials, balloons of different volumes (40 μl or 50 μl) were inflated in the experimental groups, whereas no balloons were inflated in the control group. The experimental groups underwent the first decompression in the rostral or caudal area at 1 week post-injury; the second decompression was performed at 2 weeks post-injury. For 6 weeks post-injury, the animals were tested to determine behavioral scores, somatosensory evoked potentials (SEPs) and radiographic imaging changes; histological and apoptosis assay results were subsequently analyzed.
Results: The behavioral test results and onset latency of the SEPs indicated that there were significant differences between priority rostral decompression (PRD) and priority caudal decompression (PCD) in the 50-μl compression group at 6 weeks post-injury; however, there were no significant differences between the two procedures in the 40-μl group at the same time point. Moreover, there were no significant peak-to-peak amplitude differences between the two procedures in the 50-μl compression group.
Conclusions: The findings of this study suggested that preferential rostral decompression was more beneficial than priority caudal decompression with respect to facilitating spinal cord functional recovery in rabbits with severe paraplegia and may provide clinicians with a reference for the clinical treatment of multiple-segment spinal cord compression injuries.
Keywords: Balloon compression; Decompression surgery; Multilevel spine injuries; Somatosensory evoked potentials.
Figures








Similar articles
-
Repeated injections of human umbilical cord blood-derived mesenchymal stem cells significantly promotes functional recovery in rabbits with spinal cord injury of two noncontinuous segments.Stem Cell Res Ther. 2018 May 11;9(1):136. doi: 10.1186/s13287-018-0879-0. Stem Cell Res Ther. 2018. PMID: 29751769 Free PMC article.
-
[Human umbilical cord mesenchymal stem cell transplantation for the treatment of two noncontinuous segments spinal cord compression injury in rabbits].Zhonghua Yi Xue Za Zhi. 2017 Aug 8;97(30):2366-2371. doi: 10.3760/cma.j.issn.0376-2491.2017.30.011. Zhonghua Yi Xue Za Zhi. 2017. PMID: 28822456 Chinese.
-
Somatosensory evoked potential changes and decompression timing for spinal cord function recovery and evoked potentials in rats with spinal cord injury.Brain Res Bull. 2019 Mar;146:7-11. doi: 10.1016/j.brainresbull.2018.12.003. Epub 2018 Dec 11. Brain Res Bull. 2019. PMID: 30550848
-
Indirect decompression in spinal surgery.J Clin Neurosci. 2017 Oct;44:63-68. doi: 10.1016/j.jocn.2017.06.061. Epub 2017 Jul 5. J Clin Neurosci. 2017. PMID: 28688624 Review.
-
Spinal Meninges and Their Role in Spinal Cord Injury: A Neuroanatomical Review.J Neurotrauma. 2018 Feb 1;35(3):403-410. doi: 10.1089/neu.2017.5215. Epub 2017 Oct 27. J Neurotrauma. 2018. PMID: 28922957 Review.
Cited by
-
Animal models of compression spinal cord injury.J Neurosci Res. 2022 Dec;100(12):2201-2212. doi: 10.1002/jnr.25120. Epub 2022 Sep 19. J Neurosci Res. 2022. PMID: 36121155 Free PMC article. Review.
-
Changes in neurological and pathological outcomes in a modified rat spinal cord injury model with closed canal.Neural Regen Res. 2020 Apr;15(4):697-704. doi: 10.4103/1673-5374.266919. Neural Regen Res. 2020. PMID: 31638094 Free PMC article.
-
Integration of multiple prognostic predictors in a porcine spinal cord injury model: A further step closer to reality.Front Neurol. 2023 Mar 8;14:1136267. doi: 10.3389/fneur.2023.1136267. eCollection 2023. Front Neurol. 2023. PMID: 36970513 Free PMC article.
-
Repeated injections of human umbilical cord blood-derived mesenchymal stem cells significantly promotes functional recovery in rabbits with spinal cord injury of two noncontinuous segments.Stem Cell Res Ther. 2018 May 11;9(1):136. doi: 10.1186/s13287-018-0879-0. Stem Cell Res Ther. 2018. PMID: 29751769 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

