Tissue-type plasminogen activator exerts EGF-like chemokinetic effects on oligodendrocytes in white matter (re)myelination
- PMID: 28231842
- PMCID: PMC5322587
- DOI: 10.1186/s13024-017-0160-5
Tissue-type plasminogen activator exerts EGF-like chemokinetic effects on oligodendrocytes in white matter (re)myelination
Abstract
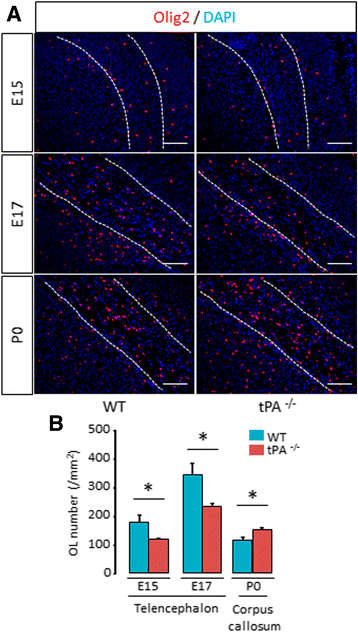
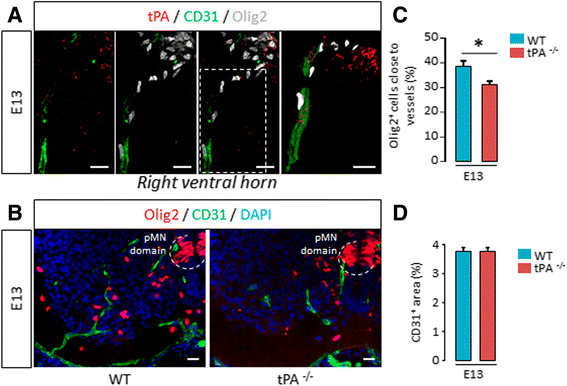
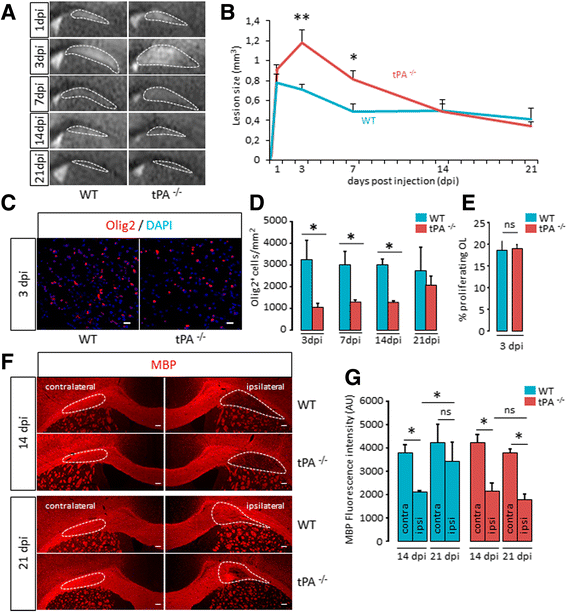
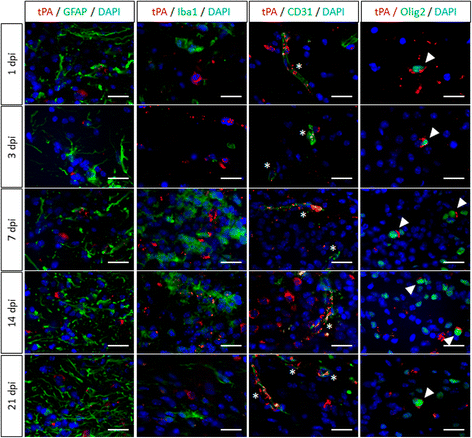
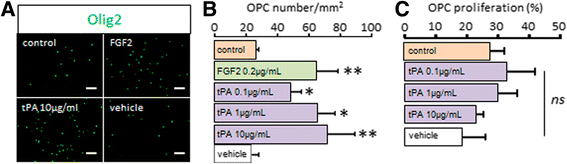
Background: The ability of oligodendrocyte progenitor cells (OPCs) to give raise to myelin forming cells during developmental myelination, normal adult physiology and post-lesion remyelination in white matter depends on factors which govern their proliferation, migration and differentiation. Tissue plasminogen activator (tPA) is a serine protease expressed in the central nervous system (CNS), where it regulates cell fate. In particular, tPA has been reported to protect oligodendrocytes from apoptosis and to facilitate the migration of neurons. Here, we investigated whether tPA can also participate in the migration of OPCs during CNS development and during remyelination after focal white matter lesion.
Methods: OPC migration was estimated by immunohistological analysis in spinal cord and corpus callosum during development in mice embryos (E13 to P0) and after white matter lesion induced by the stereotactic injection of lysolecithin in adult mice (1 to 21 days post injection). Migration was compared in these conditions between wild type and tPA knock-out animals. The action of tPA was further investigated in an in vitro chemokinesis assay.
Results: OPC migration along vessels is delayed in tPA knock-out mice during development and during remyelination. tPA enhances OPC migration via an effect dependent on the activation of epidermal growth factor receptor.
Conclusion: Endogenous tPA facilitates the migration of OPCs during development and during remyelination after white matter lesion by the virtue of its epidermal growth factor-like domain.
Keywords: Development; Endothelial cells; Epidermal growth factor; Lysolecithin; Multiple sclerosis; Myelin; Spinal cord; Vasophilic migration.
Figures




Similar articles
-
A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination.Glia. 2013 May;61(5):732-49. doi: 10.1002/glia.22469. Epub 2013 Feb 26. Glia. 2013. PMID: 23440860
-
NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration.Glia. 2013 Dec;61(12):2078-99. doi: 10.1002/glia.22578. Epub 2013 Oct 7. Glia. 2013. PMID: 24123220
-
Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination.J Neurosci. 2018 Feb 14;38(7):1802-1820. doi: 10.1523/JNEUROSCI.1291-17.2018. Epub 2018 Jan 15. J Neurosci. 2018. PMID: 29335358 Free PMC article.
-
From precursors to myelinating oligodendrocytes: contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain.Neuroscience. 2014 Jun 6;269:343-66. doi: 10.1016/j.neuroscience.2014.03.063. Epub 2014 Apr 8. Neuroscience. 2014. PMID: 24721734 Review.
-
Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor.Brain Res Rev. 2011 Jun 24;67(1-2):147-56. doi: 10.1016/j.brainresrev.2011.01.001. Epub 2011 Jan 12. Brain Res Rev. 2011. PMID: 21236296 Free PMC article. Review.
Cited by
-
Targeting mitophagy for neurological disorders treatment: advances in drugs and non-drug approaches.Naunyn Schmiedebergs Arch Pharmacol. 2023 Dec;396(12):3503-3528. doi: 10.1007/s00210-023-02636-w. Epub 2023 Aug 3. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37535076 Review.
-
Parvalbumin interneuron-derived tissue-type plasminogen activator shapes perineuronal net structure.BMC Biol. 2022 Oct 5;20(1):218. doi: 10.1186/s12915-022-01419-8. BMC Biol. 2022. PMID: 36199089 Free PMC article.
-
Extrinsic Factors Driving Oligodendrocyte Lineage Cell Progression in CNS Development and Injury.Neurochem Res. 2020 Mar;45(3):630-642. doi: 10.1007/s11064-020-02967-7. Epub 2020 Jan 29. Neurochem Res. 2020. PMID: 31997102 Free PMC article. Review.
-
Tissue plasminogen activator promotes white matter integrity and functional recovery in a murine model of traumatic brain injury.Proc Natl Acad Sci U S A. 2018 Sep 25;115(39):E9230-E9238. doi: 10.1073/pnas.1810693115. Epub 2018 Sep 10. Proc Natl Acad Sci U S A. 2018. PMID: 30201709 Free PMC article.
-
New Epidermal-Growth-Factor-Related Insights Into the Pathogenesis of Multiple Sclerosis: Is It Also Epistemology?Front Neurol. 2021 Nov 26;12:754270. doi: 10.3389/fneur.2021.754270. eCollection 2021. Front Neurol. 2021. PMID: 34899572 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

