Microbiota activates IMD pathway and limits Sindbis infection in Aedes aegypti
- PMID: 28231846
- PMCID: PMC5324288
- DOI: 10.1186/s13071-017-2040-9
Microbiota activates IMD pathway and limits Sindbis infection in Aedes aegypti
Abstract
Background: Aedes aegypti is the main vector of important arboviruses such as dengue, Zika and chikungunya. During infections mosquitoes can activate the immune pathways Toll, IMD and JAK/STAT to limit pathogen replication.
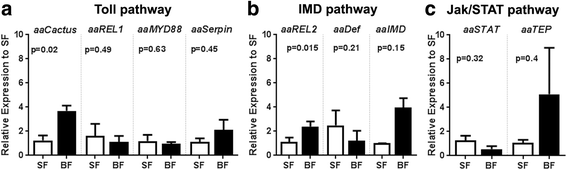
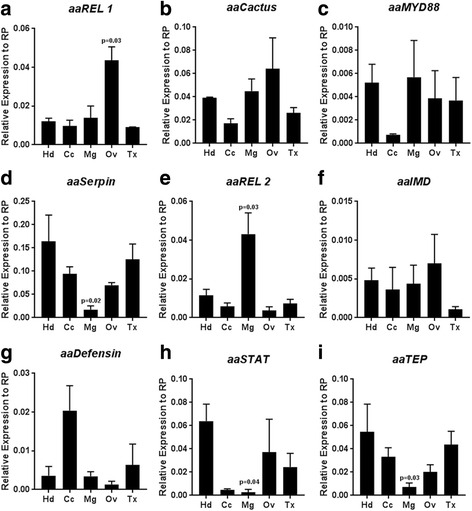
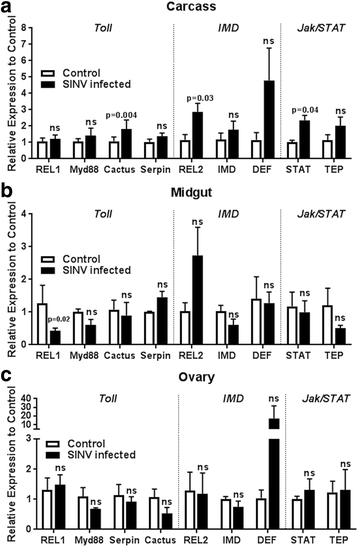
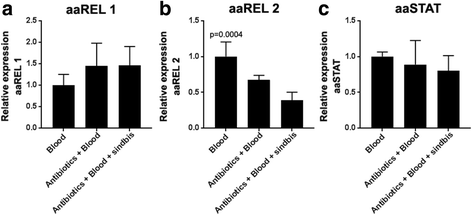
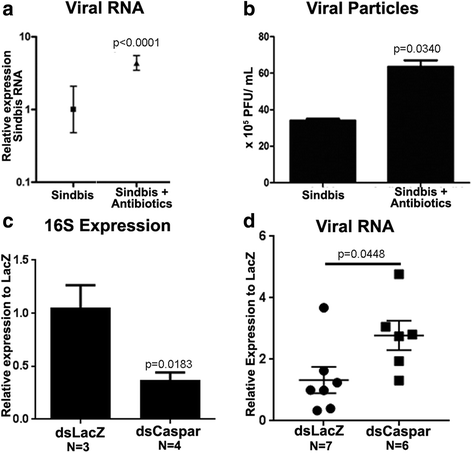
Results: Here, we evaluate the immune response profile of Ae. aegypti against Sindbis virus (SINV). We analyzed gene expression of components of Toll, IMD and JAK/STAT pathways and showed that a blood meal and virus infection upregulated aaREL2 in a microbiota-dependent fashion, since this induction was prevented by antibiotic. The presence of the microbiota activates IMD and impaired the replication of SINV in the midgut. Constitutive activation of the IMD pathway, by Caspar depletion, leads to a decrease in microbiota levels and an increase in SINV loads.
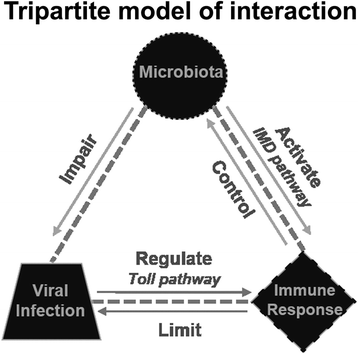
Conclusion: Together, these results suggest that a blood meal is able to activate innate immune pathways, through a nutrient induced growth of microbiota, leading to upregulation of aaREL2 and IMD activation. Microbiota levels seemed to have a reciprocal interaction, where the proliferation of the microbiota activates IMD pathway that in turn controls bacterial levels, allowing SINV replication in Ae. aegypti mosquitoes. The activation of the IMD pathway seems to have an indirect effect in SINV levels that is induced by the microbiota.
Keywords: Aedes aegypti; IMD; Immune response; Microbiota; Sindbis virus.
Figures
Similar articles
-
Effect of larval density and Sindbis virus infection on immune responses in Aedes aegypti.J Insect Physiol. 2013 Jun;59(6):604-10. doi: 10.1016/j.jinsphys.2013.03.010. Epub 2013 Apr 3. J Insect Physiol. 2013. PMID: 23562781
-
The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in Aedes aegypti.BMC Microbiol. 2010 Apr 28;10:130. doi: 10.1186/1471-2180-10-130. BMC Microbiol. 2010. PMID: 20426860 Free PMC article.
-
Transgene-mediated suppression of the RNA interference pathway in Aedes aegypti interferes with gene silencing and enhances Sindbis virus and dengue virus type 2 replication.Insect Mol Biol. 2013 Feb;22(1):104-14. doi: 10.1111/imb.12008. Insect Mol Biol. 2013. PMID: 23331493 Free PMC article.
-
Role of the Microbiome in Aedes spp. Vector Competence: What Do We Know?Viruses. 2023 Mar 17;15(3):779. doi: 10.3390/v15030779. Viruses. 2023. PMID: 36992487 Free PMC article. Review.
-
Non-immune Traits Triggered by Blood Intake Impact Vectorial Competence.Front Physiol. 2021 Mar 2;12:638033. doi: 10.3389/fphys.2021.638033. eCollection 2021. Front Physiol. 2021. PMID: 33737885 Free PMC article. Review.
Cited by
-
Multiple mosquito AMPs are needed to potentiate their antifungal effect against entomopathogenic fungi.Front Microbiol. 2023 Jan 6;13:1062383. doi: 10.3389/fmicb.2022.1062383. eCollection 2022. Front Microbiol. 2023. PMID: 36687607 Free PMC article.
-
Immune Reactions of Vector Insects to Parasites and Pathogens.Microorganisms. 2024 Mar 12;12(3):568. doi: 10.3390/microorganisms12030568. Microorganisms. 2024. PMID: 38543619 Free PMC article. Review.
-
Molecular Responses to the Zika Virus in Mosquitoes.Pathogens. 2018 May 3;7(2):49. doi: 10.3390/pathogens7020049. Pathogens. 2018. PMID: 29751526 Free PMC article. Review.
-
Aedes mosquitoes in the emerging threat of urban yellow fever transmission.Rev Med Virol. 2022 Jul;32(4):e2333. doi: 10.1002/rmv.2333. Epub 2022 Feb 6. Rev Med Virol. 2022. PMID: 35124859 Free PMC article. Review.
-
Dengue-1 virus and vector competence of Aedes aegypti (Diptera: Culicidae) populations from New Caledonia.Parasit Vectors. 2017 Aug 9;10(1):381. doi: 10.1186/s13071-017-2319-x. Parasit Vectors. 2017. PMID: 28793920 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

