COX-2 expression positively correlates with PD-L1 expression in human melanoma cells
- PMID: 28231855
- PMCID: PMC5324267
- DOI: 10.1186/s12967-017-1150-7
COX-2 expression positively correlates with PD-L1 expression in human melanoma cells
Abstract
Background: The resistance to PD-1/PD-L1 inhibitors for the treatment of melanoma have prompted investigators to implement novel clinical trials which combine immunotherapy with different treatment modalities. Moreover is also important to investigate the mechanisms which regulate the dynamic expression of PD-L1 on tumor cells and PD-1 on T cells in order to identify predictive biomarkers of response. COX-2 is currently investigated as a major player of tumor progression in several type of malignancies including melanoma. In the present study we investigated the potential relationship between COX-2 and PD-L1 expression in melanoma.
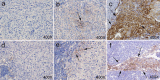
Methods: Tumor samples obtained from primary melanoma lesions and not matched lymph node metastases were analyzed for both PD-L1 and COX-2 expression by IHC analysis. Status of BRAF and NRAS mutations was analyzed by sequencing and PCR. Co-localization of PD-L1 and COX-2 expression was analyzed by double fluorescence staining. Lastly the BRAFV600E A375 and NRASQ61R SK-MEL-2 melanoma cell lines were used to evaluate the effect of COX-2 inhibition by celecoxib on expression of PD-L1 in vitro.
Results: BRAFV600E/V600K and NRASQ61R/Q61L were detected in 57.8 and 8.9% of the metastatic lesions, and in 65.9 and 6.8% of the primary tumors, respectively. PD-L1 and COX-2 expression were heterogeneously expressed in both primary melanoma lesions and not matched lymph node metastases. A significantly lower number of PD-L1 negative lesions was found in primary tumors as compared to not matched metastatic lesions (P = 0.002). COX-2 expression significantly correlated with PD-L1 expression in both primary (P = 0.001) and not matched metastatic (P = 0.048) lesions. Furthermore, in melanoma tumors, cancer cells expressing a higher levels of COX-2 also co-expressed a higher level of PD-L1. Lastly, inhibition of COX-2 activity by celecoxib down-regulated the expression of PD-L1 in both BRAFV600E A375 and NRASQ61R SK-MEL-2 melanoma cell lines.
Conclusions: COX-2 expression correlates with and modulates PD-L1 expression in melanoma cells. These findings have clinical relevance since they provide a rationale to implement novel clinical trials to test COX-2 inhibition as a potential treatment to prevent melanoma progression and immune evasion as well as to enhance the anti-tumor activity of PD-1/PD-L1 based immunotherapy for the treatment of melanoma patients with or without BRAF/NRAS mutations.
Keywords: COX-2; Celecoxib; Immune checkpoint molecules; Immunotherapy; Melanoma; PD-L1.
Figures





References
-
- McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. - DOI - PMC - PubMed
-
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

