Peptide regulation of cofilin activity in the CNS: A novel therapeutic approach for treatment of multiple neurological disorders
- PMID: 28232023
- PMCID: PMC5466456
- DOI: 10.1016/j.pharmthera.2017.02.031
Peptide regulation of cofilin activity in the CNS: A novel therapeutic approach for treatment of multiple neurological disorders
Abstract
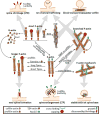
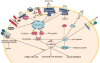
Cofilin is a ubiquitous protein which cooperates with many other actin-binding proteins in regulating actin dynamics. Cofilin has essential functions in nervous system development including neuritogenesis, neurite elongation, growth cone pathfinding, dendritic spine formation, and the regulation of neurotransmission and spine function, components of synaptic plasticity essential for learning and memory. Cofilin's phosphoregulation is a downstream target of many transmembrane signaling processes, and its misregulation in neurons has been linked in rodent models to many different neurodegenerative and neurological disorders including Alzheimer disease (AD), aggression due to neonatal isolation, autism, manic/bipolar disorder, and sleep deprivation. Cognitive and behavioral deficits of these rodent models have been largely abrogated by modulation of cofilin activity using viral-mediated, genetic, and/or small molecule or peptide therapeutic approaches. Neuropathic pain in rats from sciatic nerve compression has also been reduced by modulating the cofilin pathway within neurons of the dorsal root ganglia. Neuroinflammation, which occurs following cerebral ischemia/reperfusion, but which also accompanies many other neurodegenerative syndromes, is markedly reduced by peptides targeting specific chemokine receptors, which also modulate cofilin activity. Thus, peptide therapeutics offer potential for cost-effective treatment of a wide variety of neurological disorders. Here we discuss some recent results from rodent models using therapeutic peptides with a surprising ability to cross the rodent blood brain barrier and alter cofilin activity in brain. We also offer suggestions as to how neuronal-specific cofilin regulation might be achieved.
Keywords: Cofilin phosphoregulation; Cognitive disorders; Dendritic spines; Neuropathic pain; Psychiatric disorders; Rodent models; Sleep deprivation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Cellular prion protein: A co-receptor mediating neuronal cofilin-actin rod formation induced by β-amyloid and proinflammatory cytokines.Prion. 2014;8(6):375-80. doi: 10.4161/pri.35504. Prion. 2014. PMID: 25426519 Free PMC article. Review.
-
Dysregulation of LIMK-1/cofilin-1 pathway: A possible basis for alteration of neuronal morphology in experimental cerebral malaria.Ann Neurol. 2017 Sep;82(3):429-443. doi: 10.1002/ana.25028. Ann Neurol. 2017. PMID: 28843047
-
Synaptotoxicity in Alzheimer's Disease Involved a Dysregulation of Actin Cytoskeleton Dynamics through Cofilin 1 Phosphorylation.J Neurosci. 2018 Nov 28;38(48):10349-10361. doi: 10.1523/JNEUROSCI.1409-18.2018. Epub 2018 Oct 19. J Neurosci. 2018. PMID: 30341179 Free PMC article.
-
Cofilin Signaling in the CNS Physiology and Neurodegeneration.Int J Mol Sci. 2021 Oct 3;22(19):10727. doi: 10.3390/ijms221910727. Int J Mol Sci. 2021. PMID: 34639067 Free PMC article. Review.
-
Cofilin rod formation in neurons impairs neuronal structure and function.CNS Neurol Disord Drug Targets. 2015;14(4):554-60. doi: 10.2174/1871527314666150225144052. CNS Neurol Disord Drug Targets. 2015. PMID: 25714964 Review.
Cited by
-
Cofilin-actin rod formation in neuronal processes after brain ischemia.PLoS One. 2018 Oct 16;13(10):e0198709. doi: 10.1371/journal.pone.0198709. eCollection 2018. PLoS One. 2018. PMID: 30325927 Free PMC article.
-
Cofilin and Actin Dynamics: Multiple Modes of Regulation and Their Impacts in Neuronal Development and Degeneration.Cells. 2021 Oct 12;10(10):2726. doi: 10.3390/cells10102726. Cells. 2021. PMID: 34685706 Free PMC article. Review.
-
Molecular Mechanisms of Non-ionotropic NMDA Receptor Signaling in Dendritic Spine Shrinkage.J Neurosci. 2020 May 6;40(19):3741-3750. doi: 10.1523/JNEUROSCI.0046-20.2020. Epub 2020 Apr 22. J Neurosci. 2020. PMID: 32321746 Free PMC article.
-
Bioenergetic and excitotoxic determinants of cofilactin rod formation.J Neurochem. 2024 May;168(5):899-909. doi: 10.1111/jnc.16065. Epub 2024 Feb 1. J Neurochem. 2024. PMID: 38299375 Free PMC article.
-
α-Synuclein-mediated mitochondrial translocation of cofilin-1 leads to oxidative stress and cell apoptosis in PD.Front Neurosci. 2024 Aug 19;18:1420507. doi: 10.3389/fnins.2024.1420507. eCollection 2024. Front Neurosci. 2024. PMID: 39224576 Free PMC article.
References
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nature Neuroscience. 2001;4:367–373. - PubMed
-
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, et al. PAK3 mutation in non-syndromic X-linked mental retardation. Nature Genetics. 1998;20:25–30. - PubMed
-
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2016;12:459–509. - PubMed
-
- Ammar MR, Kassas N, Bader MF, Vitale N. Phosphatidic acid in neuronal development: a node for membrane and cytoskeletal rearrangements. Biochemie. 2014;107:51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

