Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons
- PMID: 28232180
- PMCID: PMC5414309
- DOI: 10.1016/j.neuropharm.2017.02.019
Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons
Abstract
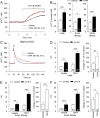
Painful nerve injury disrupts Ca2+ signaling in primary sensory neurons by elevating plasma membrane Ca2+-ATPase (PMCA) function and depressing sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) function, which decreases endoplasmic reticulum (ER) Ca2+ stores and stimulates store-operated Ca2+ entry (SOCE). The extracellular matrix glycoprotein thrombospondin-4 (TSP4), which is increased after painful nerve injury, decreases Ca2+ current (ICa) through high-voltage-activated Ca2+ channels and increases ICa through low-voltage-activated Ca2+ channels in dorsal root ganglion neurons, which are events similar to the effect of nerve injury. We therefore examined whether TSP4 plays a critical role in injury-induced disruption of intracellular Ca2+ signaling. We found that TSP4 increases PMCA activity, inhibits SERCA, depletes ER Ca2+ stores, and enhances store-operated Ca2+ influx. Injury-induced changes of SERCA and PMCA function are attenuated in TSP4 knock-out mice. Effects of TSP4 on intracellular Ca2+ signaling are attenuated in voltage-gated Ca2+ channel α2δ1 subunit (Cavα2δ1) conditional knock-out mice and are also Protein Kinase C (PKC) signaling dependent. These findings suggest that TSP4 elevation may contribute to the pathogenesis of chronic pain following nerve injury by disrupting intracellular Ca2+ signaling via interacting with the Cavα2δ1 and the subsequent PKC signaling pathway. Controlling TSP4 mediated intracellular Ca2+ signaling in peripheral sensory neurons may be a target for analgesic drug development for neuropathic pain.
Keywords: Ca(2+) stores and stimulates store-operated Ca(2+) entry; Intracellular calcium signaling; Neuropathic pain; Plasma membrane Ca(2+)-ATPase; Sarco-endoplasmic reticulum Ca(2+)-ATPase; Thrombospondin-4.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
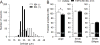







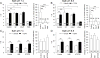

References
-
- Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. - PubMed
-
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996;66:403–411. - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

