Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17
- PMID: 28232483
- PMCID: PMC5391762
- DOI: 10.1074/jbc.M116.746859
Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17
Abstract
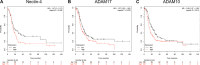
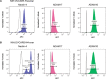
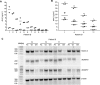
We previously showed that the cell adhesion molecule Nectin-4 is overexpressed in ovarian cancer tumors, and its cleaved extracellular domain can be detected in the serum of ovarian cancer patients. The ADAM (
Keywords: ADAM; ADAM10; ADAM17; Nectin-4; biomarker; cancer biology; cell migration; ovarian cancer.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



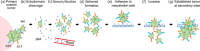
References
-
- Siegel R. L., Miller K. D., and Jemal A. (2015) Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 - PubMed
-
- Pradeep S., Kim S. W., Wu S. Y., Nishimura M., Chaluvally-Raghavan P., Miyake T., Pecot C. V., Kim S. J., Choi H. J., Bischoff F. Z., Mayer J. A., Huang L., Nick A. M., Hall C. S., Rodriguez-Aguayo C., et al. (2014) Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell 26, 77–91 - PMC - PubMed
-
- Burleson K. M., Casey R. C., Skubitz K. M., Pambuccian S. E., Oegema T. R. Jr., and Skubitz A. P. (2004) Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol. Oncol. 93, 170–181 - PubMed
-
- Burleson K. M., Hansen L. K., and Skubitz A. P. (2004) Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin. Exp. Metastasis 21, 685–697 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

