Fatty acid synthase inhibits the O- GlcNAcase during oxidative stress
- PMID: 28232487
- PMCID: PMC5399103
- DOI: 10.1074/jbc.M116.760785
Fatty acid synthase inhibits the O- GlcNAcase during oxidative stress
Abstract
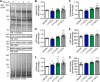
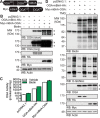
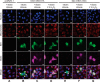
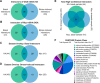
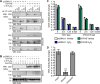
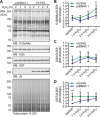
The dynamic post-translational modification O-linked β-N-acetylglucosamine (O-GlcNAc) regulates thousands of nuclear, cytoplasmic, and mitochondrial proteins. Cellular stress, including oxidative stress, results in increased O-GlcNAcylation of numerous proteins, and this increase is thought to promote cell survival. The mechanisms by which the O-GlcNAc transferase (OGT) and the O-GlcNAcase (OGA), the enzymes that add and remove O-GlcNAc, respectively, are regulated during oxidative stress to alter O-GlcNAcylation are not fully characterized. Here, we demonstrate that oxidative stress leads to elevated O-GlcNAc levels in U2OS cells but has little impact on the activity of OGT. In contrast, the expression and activity of OGA are enhanced. We hypothesized that this seeming paradox could be explained by proteins that bind to and control the local activity or substrate targeting of OGA, thereby resulting in the observed stress-induced elevations of O-GlcNAc. To identify potential protein partners, we utilized BioID proximity biotinylation in combination with
Keywords: BioID; O-GlcNAcylation; fatty acid synthase (FAS); glycoprotein; mgea5; oxidative stress; post-translational modification (PTM); protein-protein interaction; proteomics; signal transduction.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
The Nutrient-Dependent O-GlcNAc Modification Controls the Expression of Liver Fatty Acid Synthase.J Mol Biol. 2016 Aug 14;428(16):3295-3304. doi: 10.1016/j.jmb.2016.04.035. Epub 2016 May 13. J Mol Biol. 2016. PMID: 27185461
-
Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis.Int J Mol Sci. 2021 Mar 27;22(7):3463. doi: 10.3390/ijms22073463. Int J Mol Sci. 2021. PMID: 33801653 Free PMC article.
-
Elucidating the protein substrate recognition of O-GlcNAc transferase (OGT) toward O-GlcNAcase (OGA) using a GlcNAc electrophilic probe.Int J Biol Macromol. 2021 Feb 1;169:51-59. doi: 10.1016/j.ijbiomac.2020.12.078. Epub 2020 Dec 18. Int J Biol Macromol. 2021. PMID: 33333092 Free PMC article.
-
O-GlcNAc transferase and O-GlcNAcase: achieving target substrate specificity.Amino Acids. 2014 Oct;46(10):2305-16. doi: 10.1007/s00726-014-1827-7. Epub 2014 Aug 31. Amino Acids. 2014. PMID: 25173736 Free PMC article. Review.
-
O-GlcNAcase: Emerging Mechanism, Substrate Recognition and Small-Molecule Inhibitors.ChemMedChem. 2020 Jul 20;15(14):1244-1257. doi: 10.1002/cmdc.202000077. Epub 2020 Jun 16. ChemMedChem. 2020. PMID: 32496638 Review.
Cited by
-
Brain O-GlcNAcylation: From Molecular Mechanisms to Clinical Phenotype.Adv Neurobiol. 2023;29:255-280. doi: 10.1007/978-3-031-12390-0_9. Adv Neurobiol. 2023. PMID: 36255678
-
O-GlcNAcylation links oncogenic signals and cancer epigenetics.Discov Oncol. 2021 Nov 24;12(1):54. doi: 10.1007/s12672-021-00450-5. Discov Oncol. 2021. PMID: 35201498 Free PMC article. Review.
-
Spatiotemporal Proximity Labeling Tools to Track GlcNAc Sugar-Modified Functional Protein Hubs during Cellular Signaling.ACS Chem Biol. 2022 Aug 19;17(8):2153-2164. doi: 10.1021/acschembio.2c00282. Epub 2022 Jul 12. ACS Chem Biol. 2022. PMID: 35819414 Free PMC article.
-
The Emerging Roles of Protein Interactions with O-GlcNAc Cycling Enzymes in Cancer.Cancers (Basel). 2022 Oct 20;14(20):5135. doi: 10.3390/cancers14205135. Cancers (Basel). 2022. PMID: 36291918 Free PMC article. Review.
-
Liver slice culture as a model for lipid metabolism in fish.PeerJ. 2019 Sep 17;7:e7732. doi: 10.7717/peerj.7732. eCollection 2019. PeerJ. 2019. PMID: 31576253 Free PMC article.
References
-
- Lubas W. A., Frank D. W., Krause M., and Hanover J. A. (1997) O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272, 9316–9324 - PubMed
-
- Kreppel L. K., Blomberg M. A., and Hart G. W. (1997) Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

