Cognitive Implications of Deep Gray Matter Iron in Multiple Sclerosis
- PMID: 28232497
- PMCID: PMC7960387
- DOI: 10.3174/ajnr.A5109
Cognitive Implications of Deep Gray Matter Iron in Multiple Sclerosis
Abstract
Background and purpose: Deep gray matter iron accumulation is increasingly recognized in association with multiple sclerosis and can be measured in vivo with MR imaging. The cognitive implications of this pathology are not well-understood, especially vis-à-vis deep gray matter atrophy. Our aim was to investigate the relationships between cognition and deep gray matter iron in MS by using 2 MR imaging-based iron-susceptibility measures.
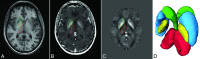
Materials and methods: Forty patients with multiple sclerosis (relapsing-remitting, n = 16; progressive, n = 24) and 27 healthy controls were imaged at 4.7T by using the transverse relaxation rate and quantitative susceptibility mapping. The transverse relaxation rate and quantitative susceptibility mapping values and volumes (atrophy) of the caudate, putamen, globus pallidus, and thalamus were determined by multiatlas segmentation. Cognition was assessed with the Brief Repeatable Battery of Neuropsychological Tests. Relationships between cognition and deep gray matter iron were examined by hierarchic regressions.
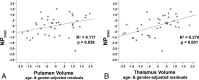
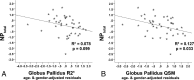
Results: Compared with controls, patients showed reduced memory (P < .001) and processing speed (P = .02) and smaller putamen (P < .001), globus pallidus (P = .002), and thalamic volumes (P < .001). Quantitative susceptibility mapping values were increased in patients compared with controls in the putamen (P = .003) and globus pallidus (P = .003). In patients only, thalamus (P < .001) and putamen (P = .04) volumes were related to cognitive performance. After we controlled for volume effects, quantitative susceptibility mapping values in the globus pallidus (P = .03; trend for transverse relaxation rate, P = .10) were still related to cognition.
Conclusions: Quantitative susceptibility mapping was more sensitive compared with the transverse relaxation rate in detecting deep gray matter iron accumulation in the current multiple sclerosis cohort. Atrophy and iron accumulation in deep gray matter both have negative but separable relationships to cognition in multiple sclerosis.
© 2017 by American Journal of Neuroradiology.
Figures

Similar articles
-
Determinants of Deep Gray Matter Atrophy in Multiple Sclerosis: A Multimodal MRI Study.AJNR Am J Neuroradiol. 2019 Jan;40(1):99-106. doi: 10.3174/ajnr.A5915. Epub 2018 Dec 20. AJNR Am J Neuroradiol. 2019. PMID: 30573464 Free PMC article.
-
Subcortical gray matter segmentation and voxel-based analysis using transverse relaxation and quantitative susceptibility mapping with application to multiple sclerosis.J Magn Reson Imaging. 2015 Dec;42(6):1601-10. doi: 10.1002/jmri.24951. Epub 2015 May 18. J Magn Reson Imaging. 2015. PMID: 25980643
-
Progressive iron accumulation across multiple sclerosis phenotypes revealed by sparse classification of deep gray matter.J Magn Reson Imaging. 2017 Nov;46(5):1464-1473. doi: 10.1002/jmri.25682. Epub 2017 Mar 16. J Magn Reson Imaging. 2017. PMID: 28301067
-
Quantitative Susceptibility Mapping Values Quantification in Deep Gray Matter Structures for Relapsing-Remitting Multiple Sclerosis: A Systematic Review and Meta-Analysis.Brain Behav. 2024 Oct;14(10):e70093. doi: 10.1002/brb3.70093. Brain Behav. 2024. PMID: 39415615 Free PMC article.
-
Quantitative susceptibility mapping of brain iron in healthy aging and cognition.Neuroimage. 2023 Nov 15;282:120401. doi: 10.1016/j.neuroimage.2023.120401. Epub 2023 Oct 5. Neuroimage. 2023. PMID: 37802405 Free PMC article. Review.
Cited by
-
Thalamic Nuclei Volumes and Their Relationships to Neuroperformance in Multiple Sclerosis: A Cross-Sectional Structural MRI Study.J Magn Reson Imaging. 2021 Mar;53(3):731-739. doi: 10.1002/jmri.27389. Epub 2020 Oct 12. J Magn Reson Imaging. 2021. PMID: 33044013 Free PMC article.
-
Neuroimaging Correlates of Cognitive Dysfunction in Adults with Multiple Sclerosis.Brain Sci. 2021 Mar 9;11(3):346. doi: 10.3390/brainsci11030346. Brain Sci. 2021. PMID: 33803287 Free PMC article. Review.
-
Iron related changes in MS lesions and their validity to characterize MS lesion types and dynamics with Ultra-high field magnetic resonance imaging.Brain Pathol. 2018 Sep;28(5):743-749. doi: 10.1111/bpa.12643. Brain Pathol. 2018. PMID: 30020556 Free PMC article. Review.
-
Regional high iron deposition on quantitative susceptibility mapping correlates with cognitive decline in type 2 diabetes mellitus.Front Neurosci. 2023 Jan 30;17:1061156. doi: 10.3389/fnins.2023.1061156. eCollection 2023. Front Neurosci. 2023. PMID: 36793541 Free PMC article.
-
Quantitative susceptibility mapping of the fear circuit: Associations with silent symptoms in relapsing-remitting multiple sclerosis.Neuroradiol J. 2025 Aug;38(4):464-474. doi: 10.1177/19714009241303123. Epub 2024 Dec 4. Neuroradiol J. 2025. PMID: 39631056 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
