GSK3-mediated CLASP2 phosphorylation modulates kinetochore dynamics
- PMID: 28232523
- PMCID: PMC5399784
- DOI: 10.1242/jcs.194662
GSK3-mediated CLASP2 phosphorylation modulates kinetochore dynamics
Abstract
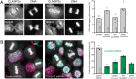
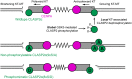
Error-free chromosome segregation requires dynamic control of microtubule attachment to kinetochores, but how kinetochore-microtubule interactions are spatially and temporally controlled during mitosis remains incompletely understood. In addition to the NDC80 microtubule-binding complex, other proteins with demonstrated microtubule-binding activities localize to kinetochores. One such protein is the cytoplasmic linker-associated protein 2 (CLASP2). Here, we show that global GSK3-mediated phosphorylation of the longest isoform, CLASP2α, largely abolishes CLASP2α-microtubule association in metaphase. However, it does not directly control localization of CLASP2α to kinetochores. Using dominant phosphorylation-site variants, we find that CLASP2α phosphorylation weakens kinetochore-microtubule interactions as evidenced by decreased tension between sister kinetochores. Expression of CLASP2α phosphorylation-site mutants also resulted in increased chromosome segregation defects, indicating that GSK3-mediated control of CLASP2α-microtubule interactions contributes to correct chromosome dynamics. Because of global inhibition of CLASP2α-microtubule interactions, we propose a model in which only kinetochore-bound CLASP2α is dephosphorylated, locally engaging its microtubule-binding activity.
Keywords: CLASP2; GSK3; Kinetochore; Microtubule; Mitosis; Phosphorylation.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




Similar articles
-
Ska3 Phosphorylated by Cdk1 Binds Ndc80 and Recruits Ska to Kinetochores to Promote Mitotic Progression.Curr Biol. 2017 May 22;27(10):1477-1484.e4. doi: 10.1016/j.cub.2017.03.060. Epub 2017 May 4. Curr Biol. 2017. PMID: 28479321
-
RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment.J Cell Sci. 2009 Jul 15;122(Pt 14):2436-45. doi: 10.1242/jcs.051912. Epub 2009 Jun 23. J Cell Sci. 2009. PMID: 19549680
-
CLASP2 binding to curved microtubule tips promotes flux and stabilizes kinetochore attachments.J Cell Biol. 2020 Feb 3;219(2):e201905080. doi: 10.1083/jcb.201905080. J Cell Biol. 2020. PMID: 31757788 Free PMC article.
-
Multitasking Ska in Chromosome Segregation: Its Distinct Pools Might Specify Various Functions.Bioessays. 2018 Mar;40(3):10.1002/bies.201700176. doi: 10.1002/bies.201700176. Epub 2018 Jan 23. Bioessays. 2018. PMID: 29359816 Free PMC article. Review.
-
Kinetochore-microtubule coupling mechanisms mediated by the Ska1 complex and Cdt1.Essays Biochem. 2020 Sep 4;64(2):337-347. doi: 10.1042/EBC20190075. Essays Biochem. 2020. PMID: 32844209 Free PMC article. Review.
Cited by
-
Circa-SCOPE: high-throughput live single-cell imaging method for analysis of circadian clock resetting.Nat Commun. 2021 Oct 8;12(1):5903. doi: 10.1038/s41467-021-26210-1. Nat Commun. 2021. PMID: 34625543 Free PMC article.
-
The mitotic protein NuMA plays a spindle-independent role in nuclear formation and mechanics.J Cell Biol. 2020 Dec 7;219(12):e202004202. doi: 10.1083/jcb.202004202. J Cell Biol. 2020. PMID: 33044554 Free PMC article.
-
Syncytia formation by SARS-CoV-2-infected cells.EMBO J. 2020 Dec 1;39(23):e106267. doi: 10.15252/embj.2020106267. Epub 2020 Nov 4. EMBO J. 2020. PMID: 33051876 Free PMC article.
-
Microtubule End-Clustering Maintains a Steady-State Spindle Shape.Curr Biol. 2019 Feb 18;29(4):700-708.e5. doi: 10.1016/j.cub.2019.01.016. Epub 2019 Feb 7. Curr Biol. 2019. PMID: 30744975 Free PMC article.
-
In vivo 3D profiling of site-specific human cancer cell morphotypes in zebrafish.J Cell Biol. 2022 Nov 7;221(11):e202109100. doi: 10.1083/jcb.202109100. Epub 2022 Sep 26. J Cell Biol. 2022. PMID: 36155740 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

