FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis
- PMID: 28232599
- PMCID: PMC5344649
- DOI: 10.1634/theoncologist.2016-0476
FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis
Abstract
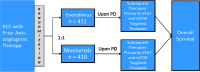
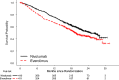
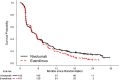
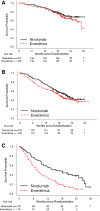
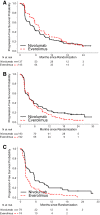
On November 23, 2015, the U.S. Food and Drug Administration approved nivolumab (OPDIVO, Bristol-Myers Squibb Company) for patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy. The approval was based on efficacy and safety data demonstrated in an open-label, randomized study of 821 patients with advanced RCC who progressed after at least one anti-angiogenic therapy. Patients were randomized to nivolumab or everolimus and followed for disease progression. The primary end point was overall survival. Subsequent therapies, including everolimus for patients who developed progressive disease on the nivolumab arm, were allowed, but no cross-over was permitted. The median overall survival was 25.0 months on the nivolumab arm and 19.6 months on everolimus arm (hazard ratio: 0.73; 95% confidence interval: 0.60-0.89). The confirmed response rates were 21.5% versus 3.9%; median durations of response were 23.0 versus 13.7 months, and median times to response were 3.0 versus 3.7 months in the nivolumab and everolimus arms, respectively. A statistically significant improvement in progression-free survival was not observed in this trial. The safety profile of nivolumab in renal cell cancer was similar to that in other disease settings. However, the incidence of immune-mediated nephritis appeared to be higher in patients with RCC. The Oncologist 2017;22:311-317 IMPLICATIONS FOR PRACTICE: The overall benefit/risk profile demonstrated in trial CA209025 supported the approval of nivolumab as an additional treatment option for patients with advanced renal cell carcinoma after anti-angiogenic therapy. The use of nivolumab in patients who had received vascular endothelial growth factor-targeted therapy resulted in a 5.4 month improvement in median overall survival compared with the everolimus arm. This difference is statistically significant and clinically meaningful.
Keywords: Advanced renal cell carcinoma; Biomarker; Immunotherapy; Nivolumab.
© AlphaMed Press 2017.
Conflict of interest statement
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. - PubMed
-
- Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. - PubMed
-
- Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: A double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008;372:449–456. - PubMed
-
- Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open‐label, multicentre trial. Lancet Oncol 2015;16:1473–1482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

