Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis
- PMID: 28232662
- PMCID: PMC5336560
- DOI: 10.1038/emm.2016.151
Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis
Abstract
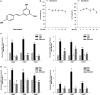
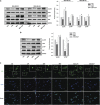
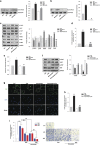
Hippo/YAP signaling is implicated in tumorigenesis and progression of various cancers. By inhibiting a plethora signaling cascades, resveratrol has strong anti-tumorigenic and anti-metastatic activity. In the present study, we demonstrate that resveratrol decreases the expression of YAP target genes. In addition, our data showed that resveratrol attenuates breast cancer cell invasion through the activation of Lats1 and consequent inactivation of YAP. Strikingly, we also demonstrate that resveratrol inactivates RhoA, leading to the activation of Lats1 and induction of YAP phosphorylation. Further, resveratrol in combination with other agents that inactivate RhoA or YAP showed more marked suppression of breast cancer cell invasion compared with single treatment. Collectively, these findings indicate the beneficial effects of resveratrol on breast cancer patients by suppressing the RhoA/Lats1/YAP signaling axis and subsequently inhibiting breast cancer cell invasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

